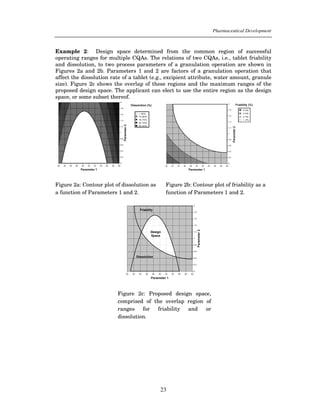
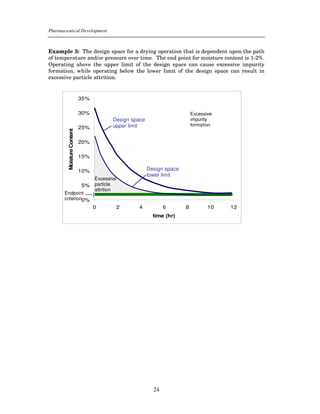
This document provides guidance on the content of the Pharmaceutical Development section (3.2.P.2) of regulatory submissions for drug products. It describes the components and attributes of drug products and manufacturing processes that should be discussed, including drug substances, excipients, formulations, manufacturing methods, and controls. The level of scientific knowledge provided in the submission forms the basis for flexible regulatory approaches. The guidance aims to facilitate a comprehensive understanding of products and processes for regulatory review and inspection.