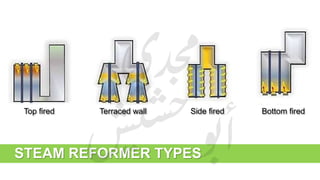
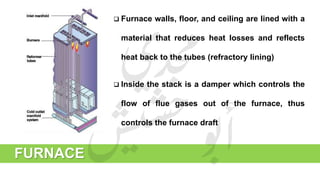
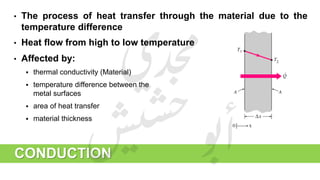
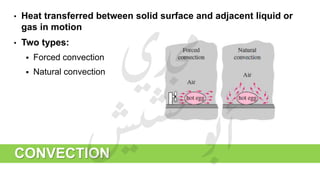
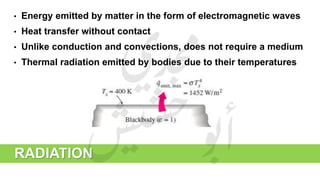
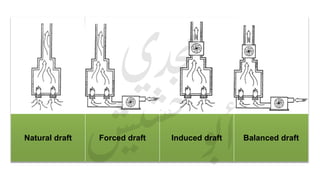
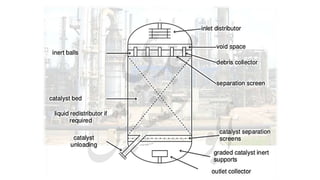
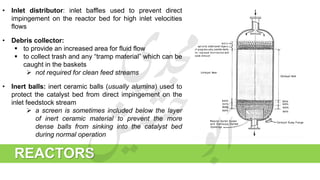
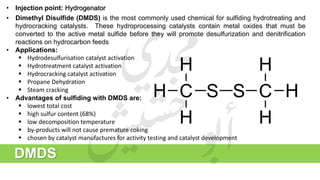
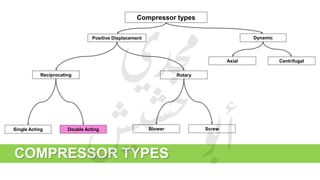
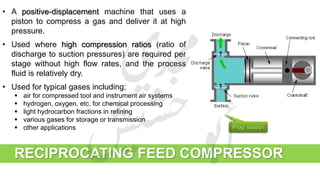
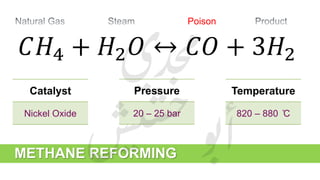
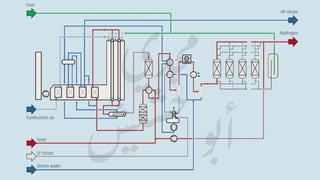
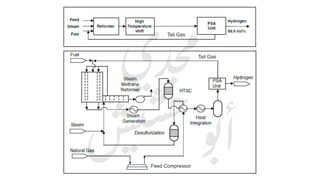
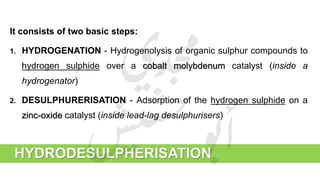
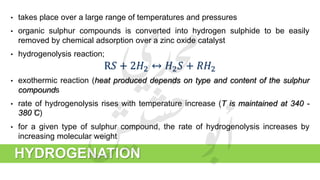
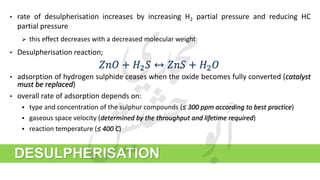
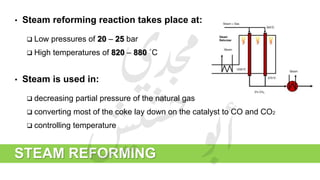
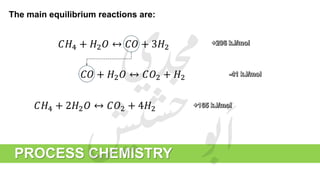
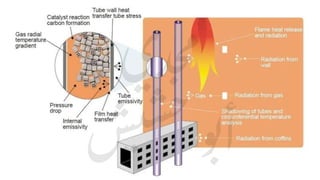
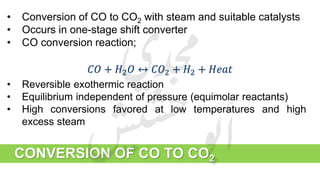
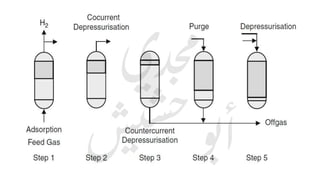
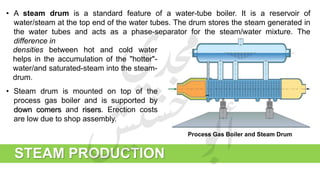
The document provides a comprehensive overview of hydrogen production and its applications in refineries, detailing key processes such as steam reforming, purification, and feed preparation. It emphasizes the importance of hydrogen for processing heavy petroleum fractions and outlines various operational parameters, equipment types, and process chemistry involved in hydrogen production. Additionally, it addresses the critical role of catalysts and compressors in enhancing efficiency and purity throughout the hydrogen generation process.