This document provides an introduction to hydrogen, including:
- Hydrogen is the lightest element and most abundant in the universe. On Earth, it exists primarily in molecular form.
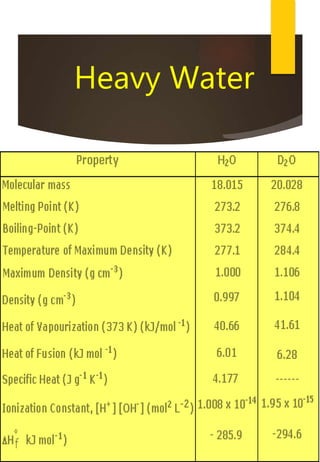
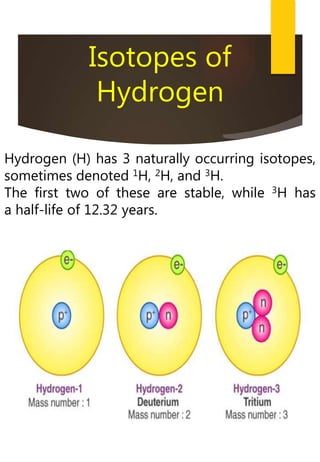
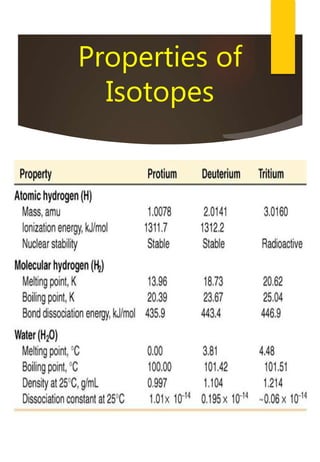
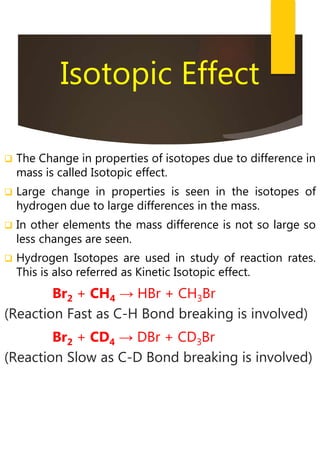
- Hydrogen has three naturally occurring isotopes: protium, deuterium, and tritium. Differences in mass between isotopes cause isotopic effects.
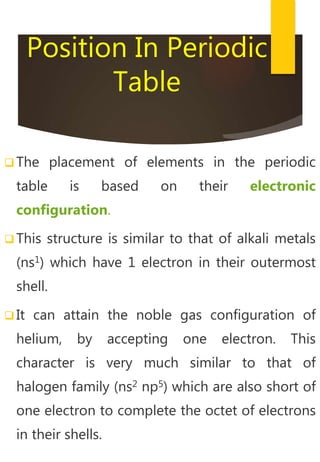
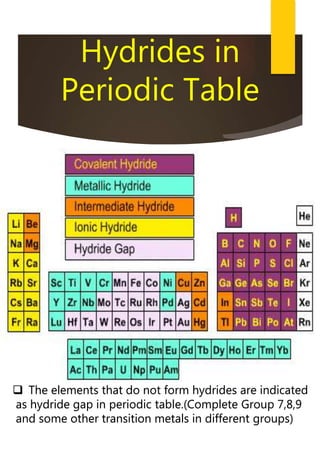
- Hydrogen exhibits properties of both alkali metals and halogens due to its electronic configuration and reactivity. It is placed in the periodic table between them.
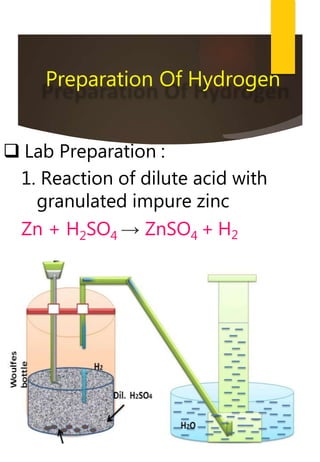
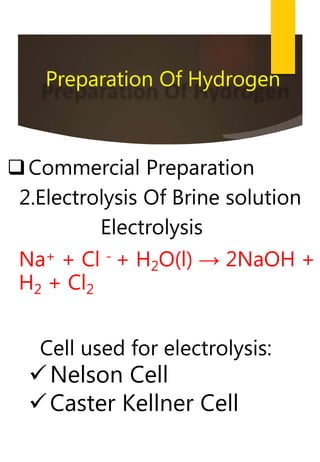
- Common methods to produce hydrogen include the electrolysis of water and the reaction of steam with hydrocarbons over a catalyst. Hydrogen has many industrial and energy applications.




























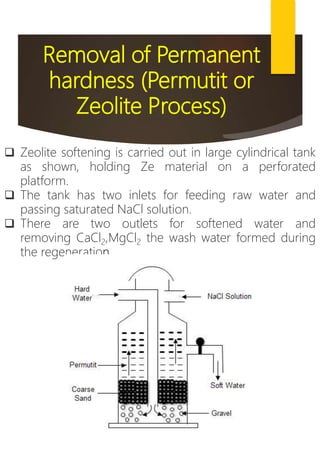
![Removal of Permanent
hardness (Calgon’s
Process)
Calgon method involves treatment of Calgon
(sodium hexametaphosphate, Na6P6O18) to
form complex anion, which keeps the Mg 2+
and Ca 2+ ions in solution
Calgon ionizes to give a complex anion:
(NaPO3)6 → 2Na+ + [Na4P6O18 ]2-
The addition of Calgon to hard water causes
the calcium and magnesium ions of hard
water to displace sodium ions from the anion
of Calgon.
Ca2++ [Na4P6O18 ]2- → 2Na+ +
[CaNa2P6O18 ]2-
Anion of calgon goes into solution This
results in the removal of calcium and
magnesium ions from hard water in the form
of a complex with Calgon.](https://image.slidesharecdn.com/hydrogenanditscompoundspankajbahrani-210303092158/85/Hydrogen-and-its-compounds-44-320.jpg)