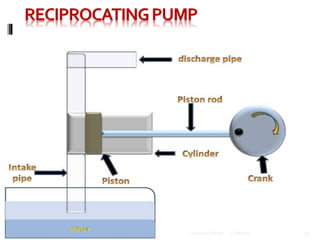
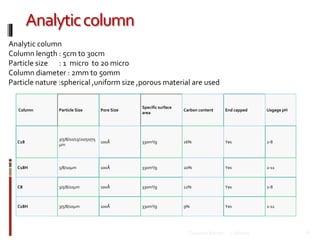
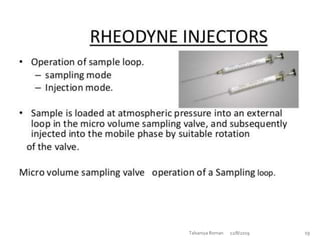
This document discusses HPLC (High Performance Liquid Chromatography). It begins by providing an introduction to Talsaniya Roman, the author, and their presentation on HPLC. It then covers various topics related to HPLC in more detail over several pages, including the definition of chromatography, different types of HPLC classification like normal phase vs reversed phase, different stationary and mobile phases, detectors commonly used in HPLC like UV, refractive index, fluorescence, and conductivity detectors, and key components of an HPLC system like the pump, injector, and column. The document provides information on HPLC in a technical presentation format.