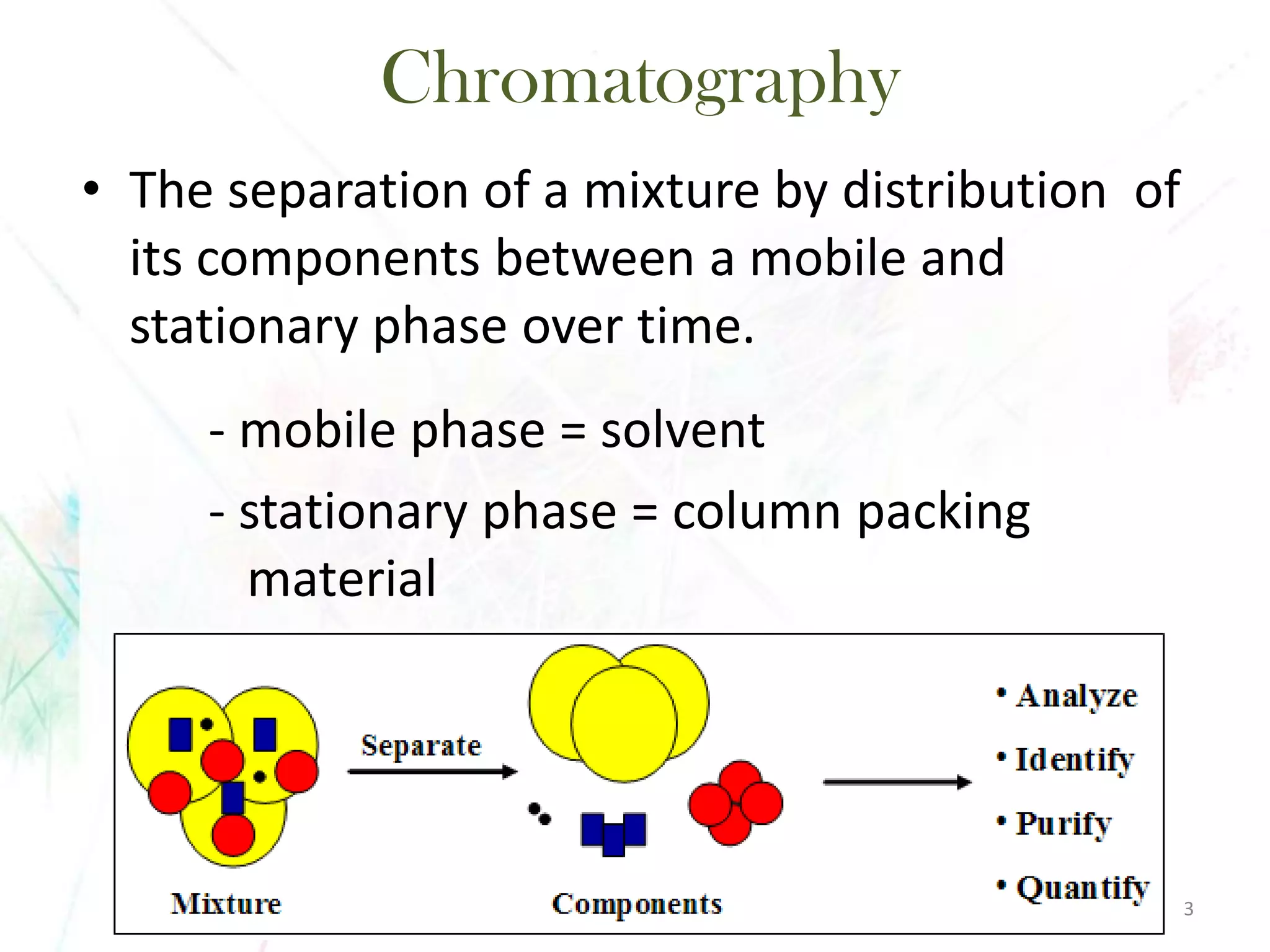
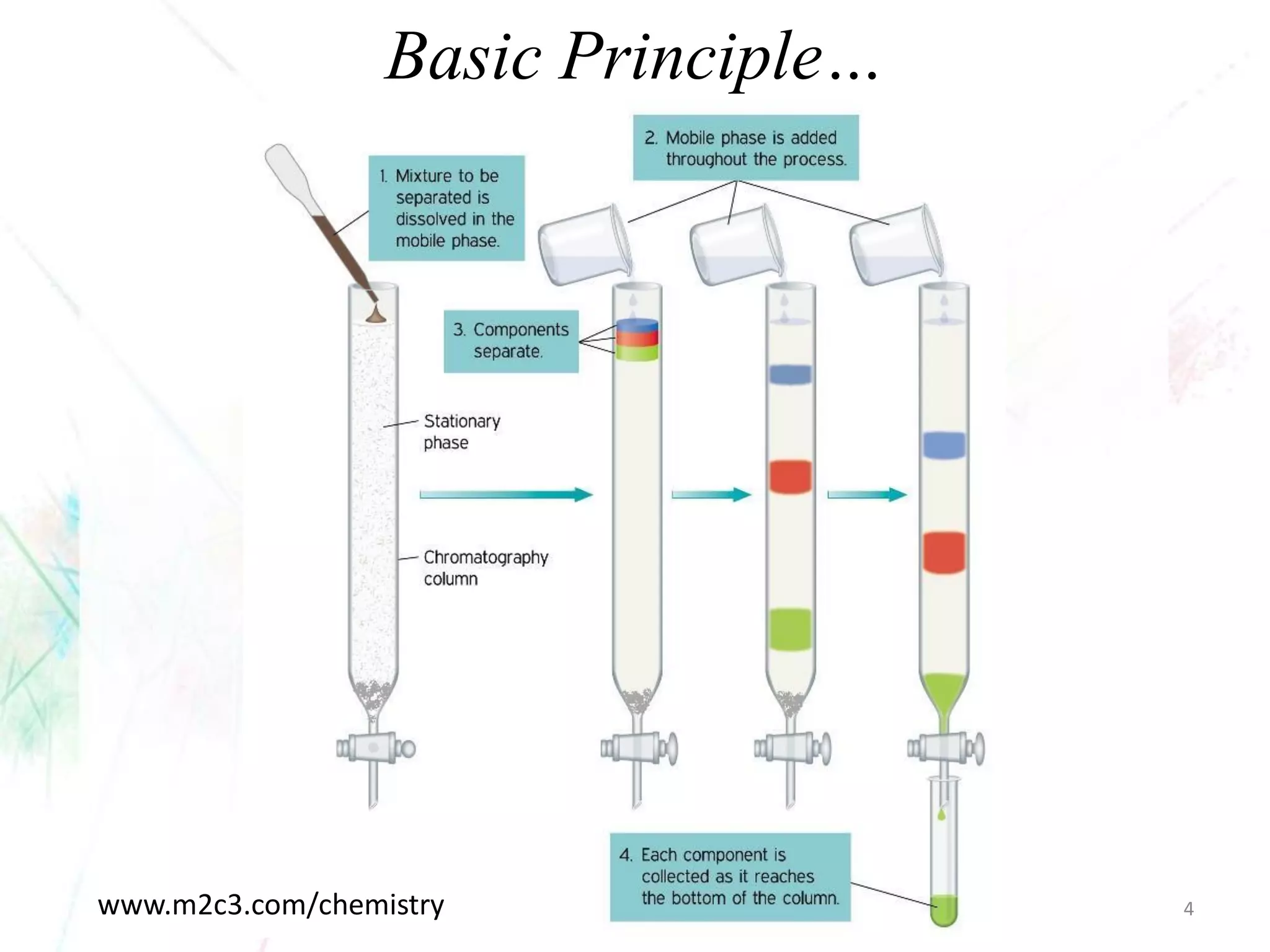
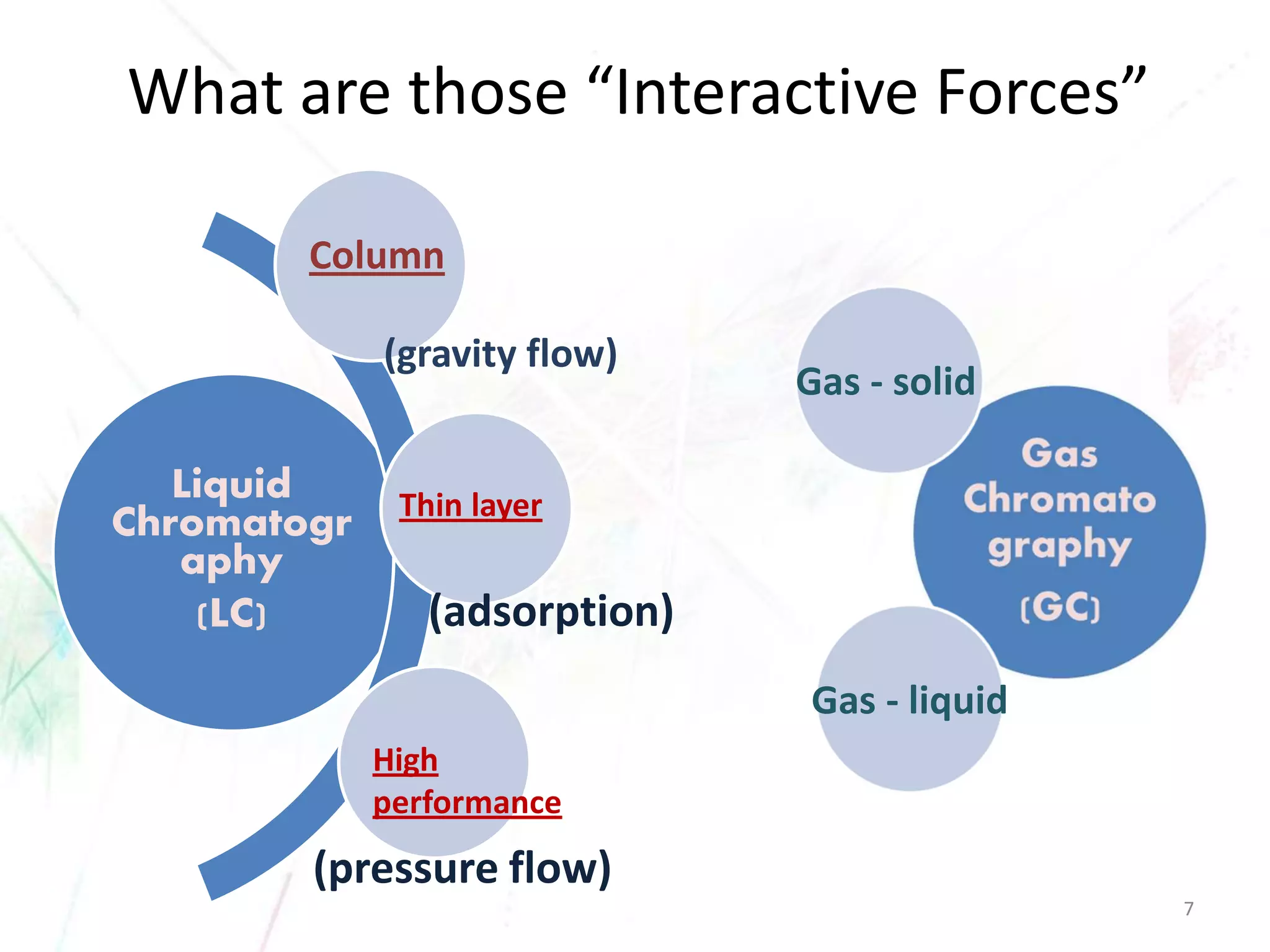
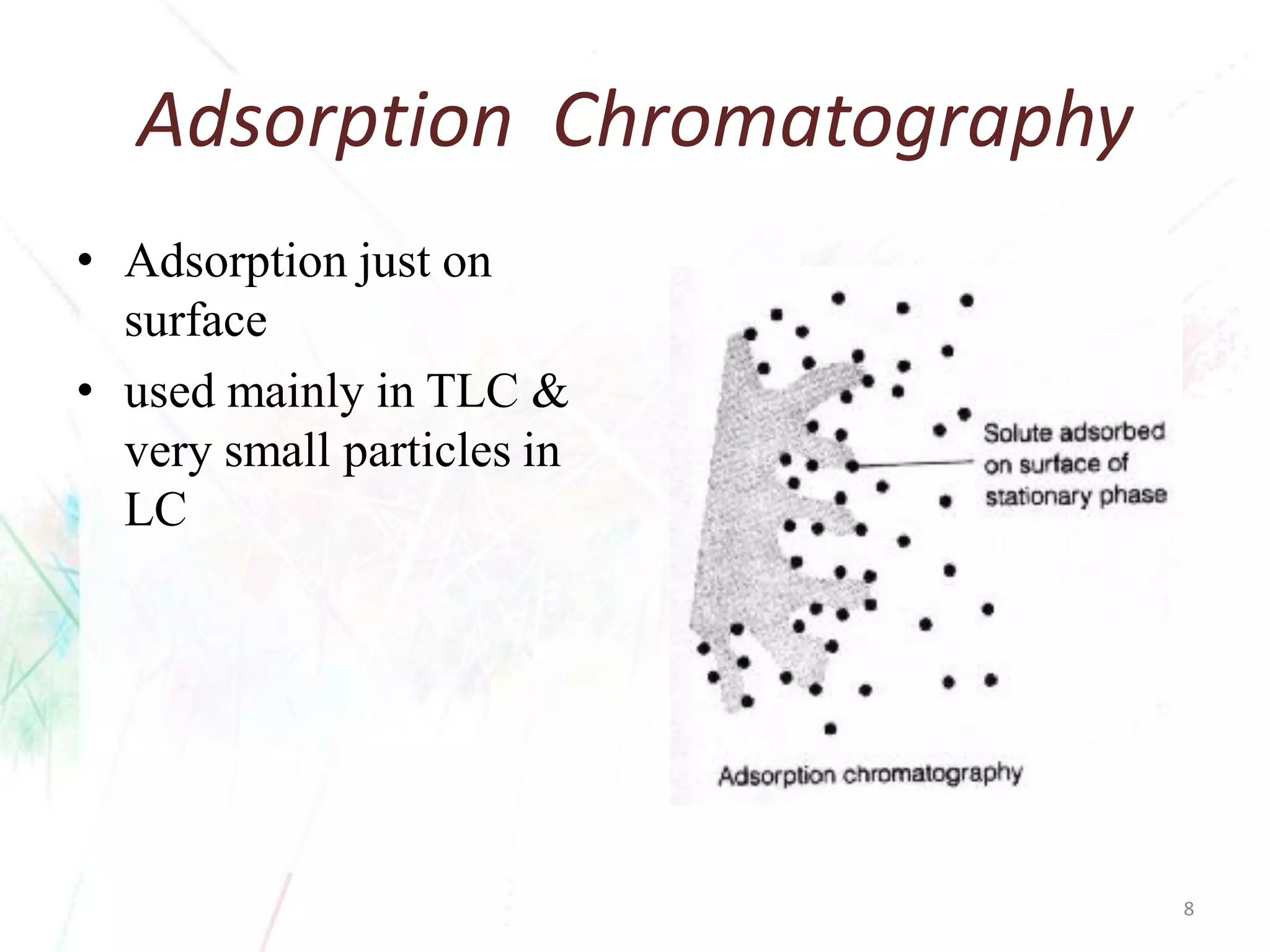
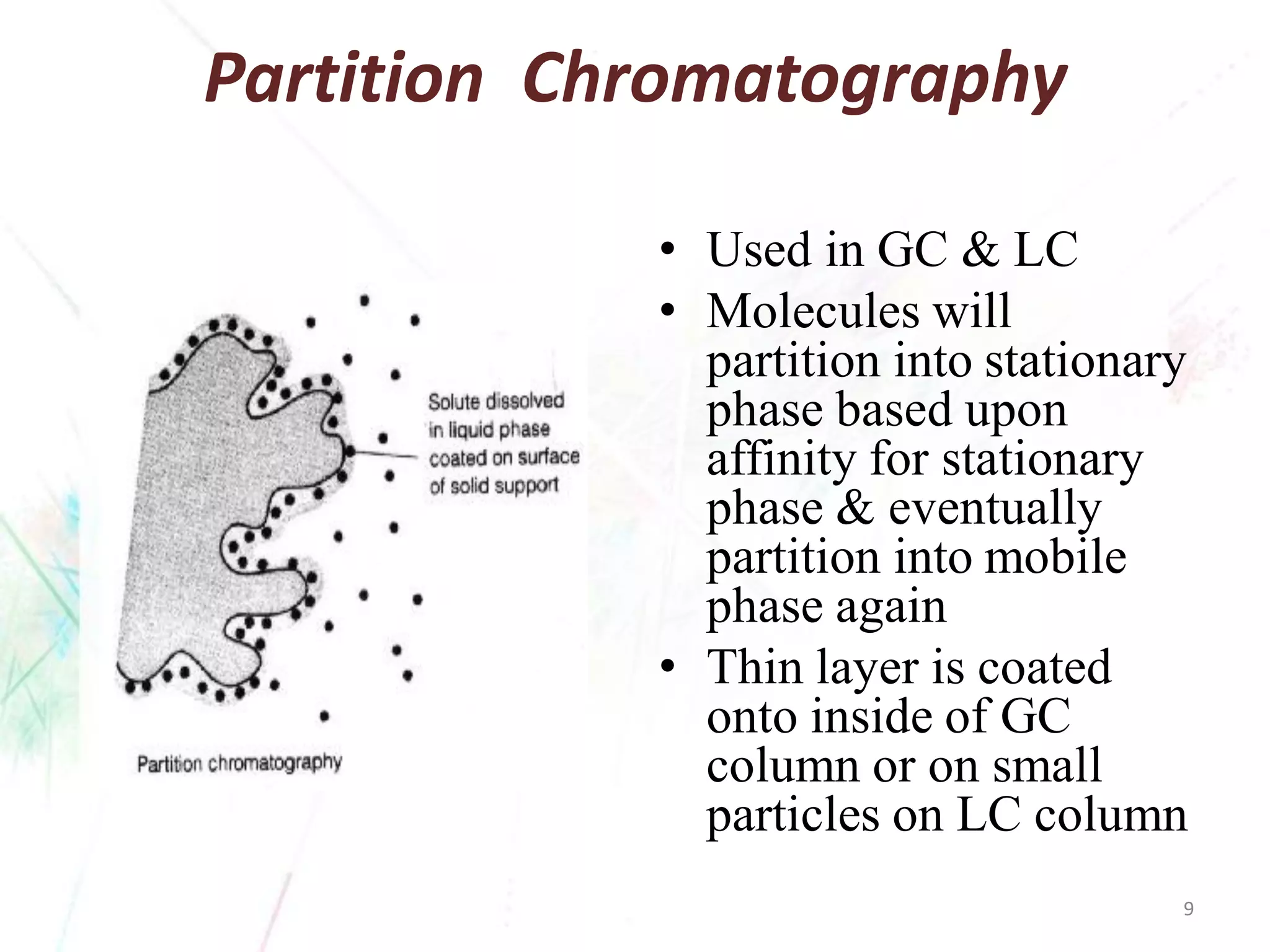
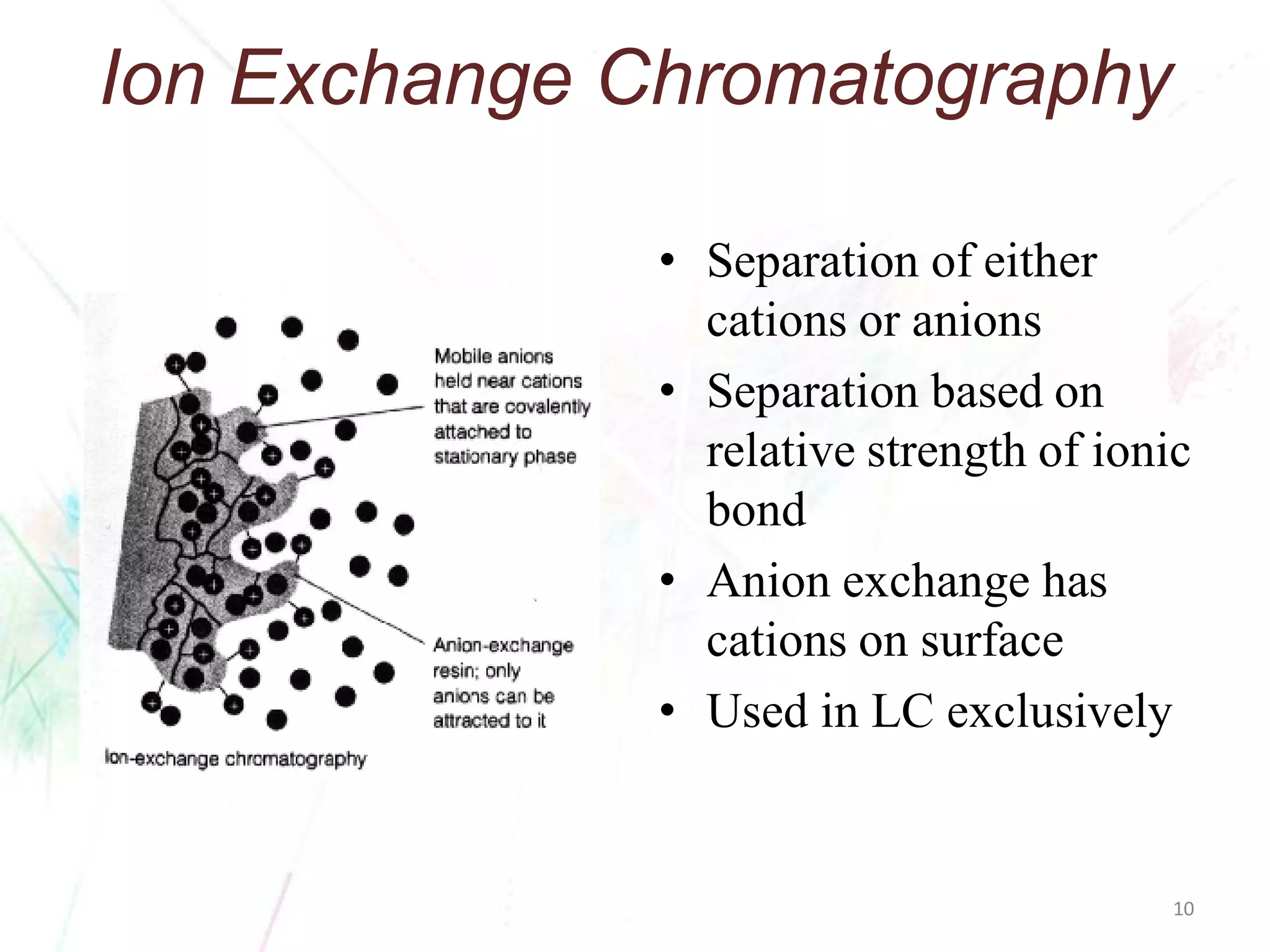
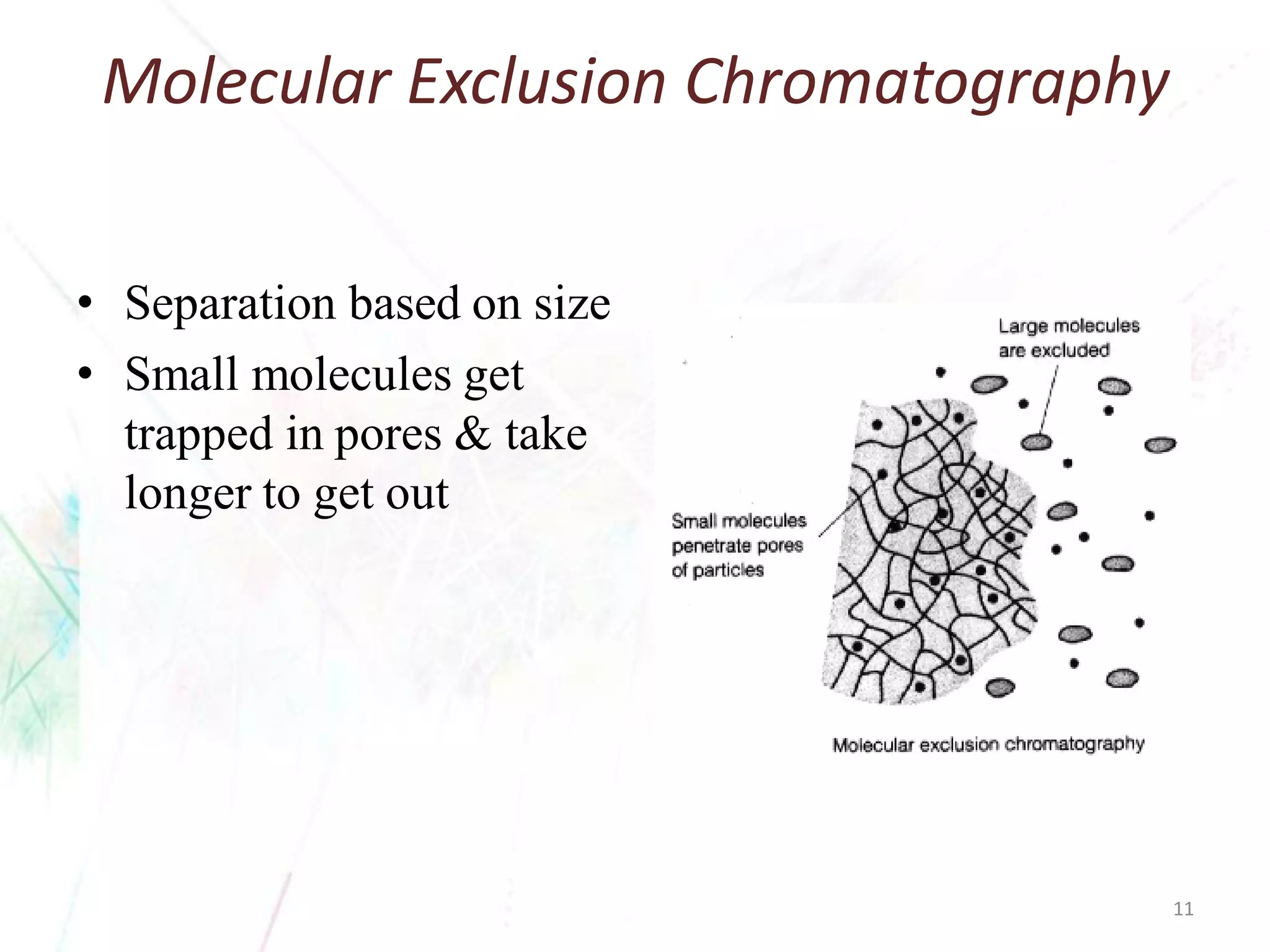
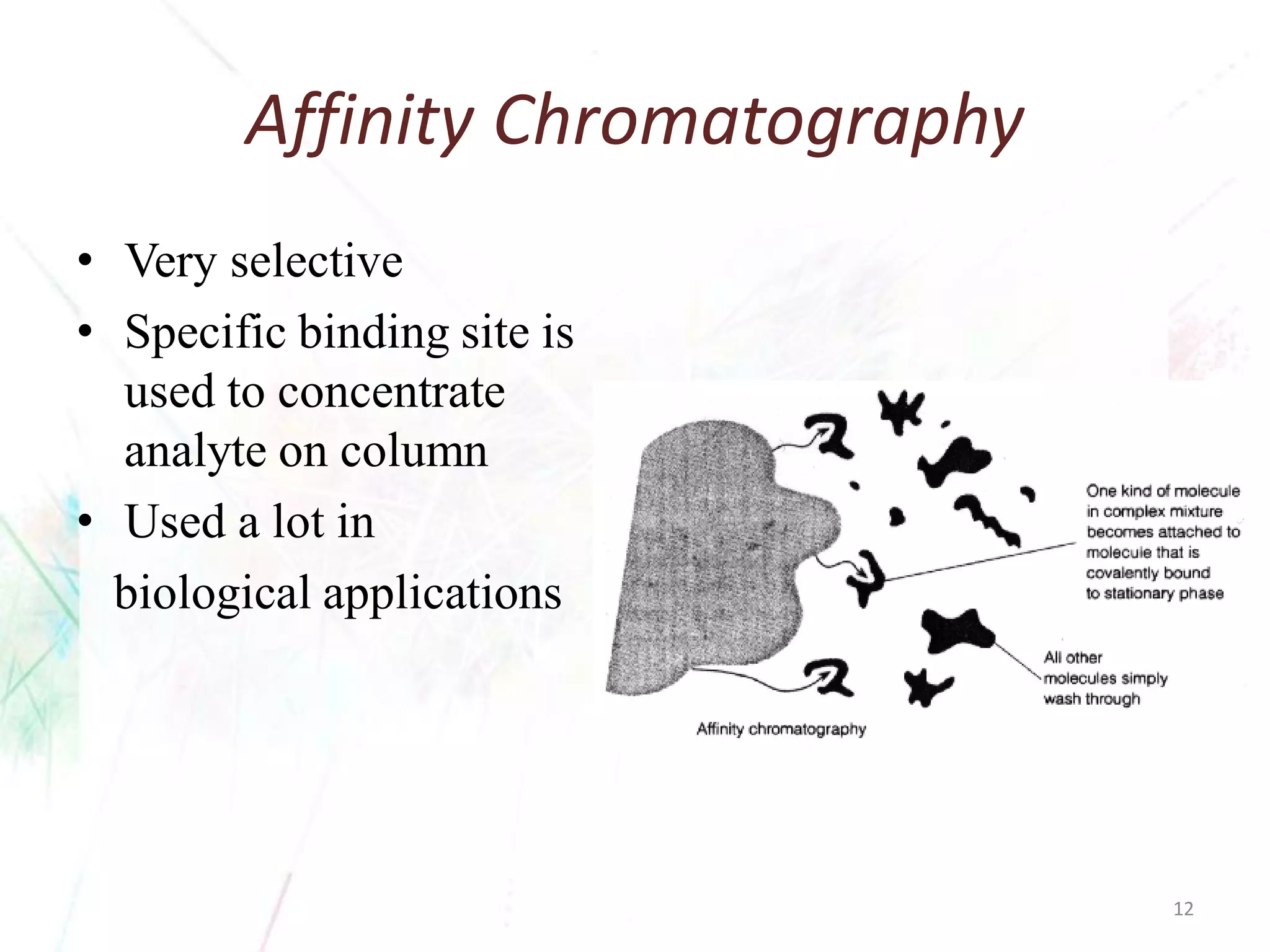
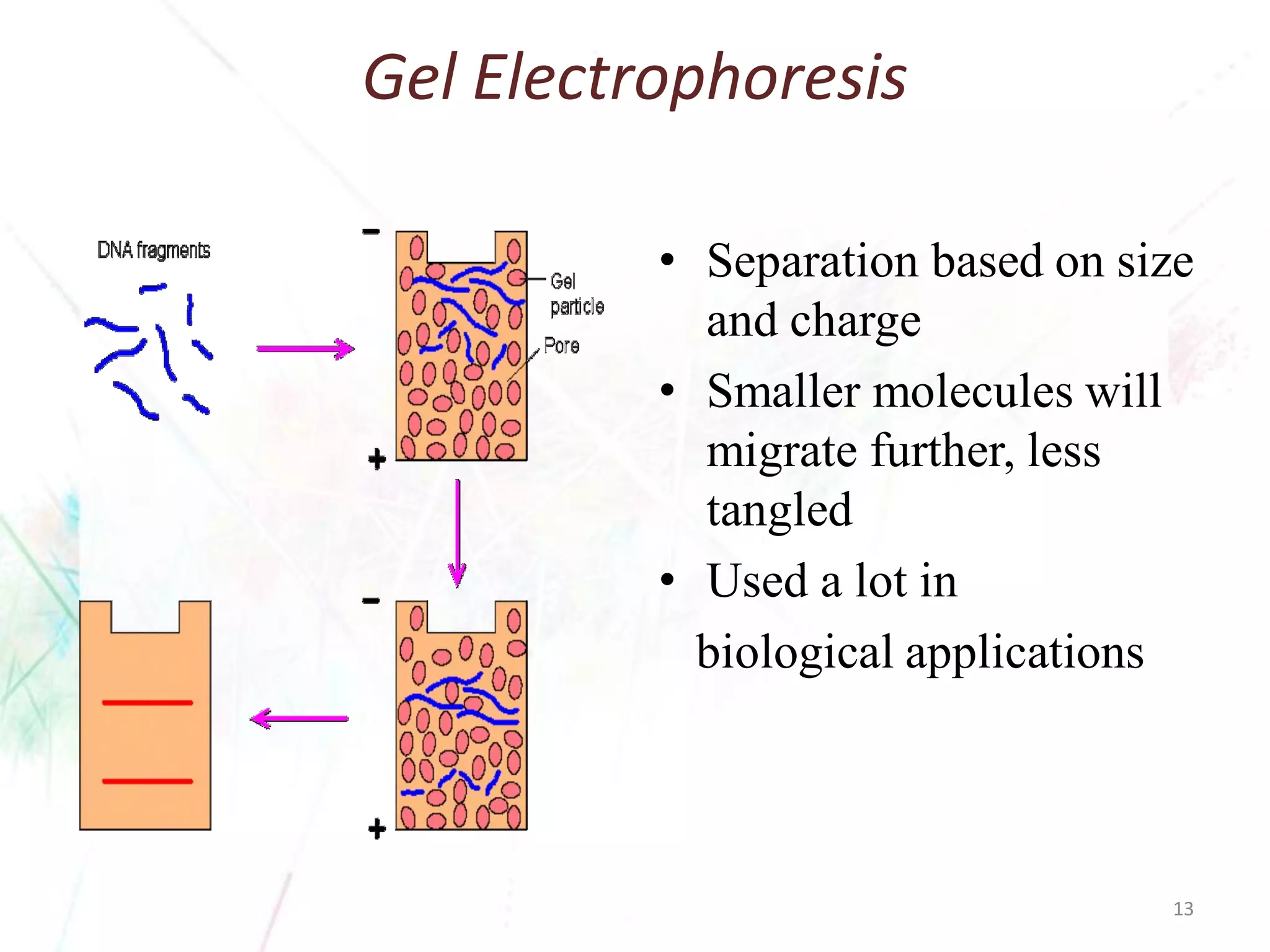
Chromatography separates the components of a mixture through differential interactions between the components and both a mobile phase and a stationary phase. Components migrate through the stationary phase at different rates depending on their varying levels of affinity or "interactive force" for each phase. This differential migration over time allows the components to be separated from one another as the mixture moves through the column. The primary interactive forces that cause separation include adsorption, partition, ion exchange, molecular size exclusion, affinity, and electrophoretic mobility.