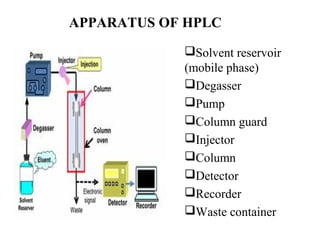
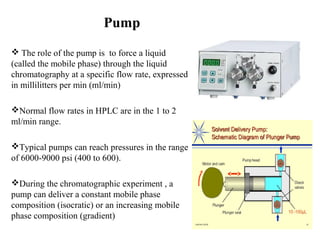
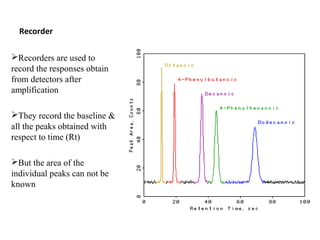
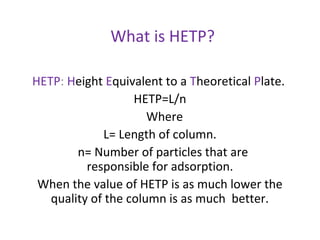
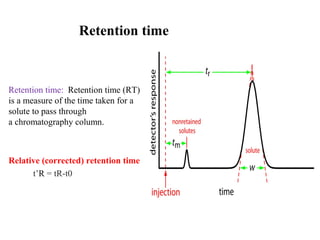
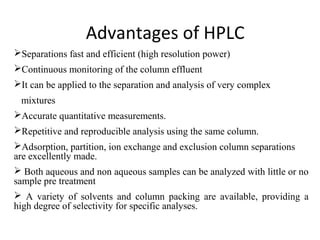
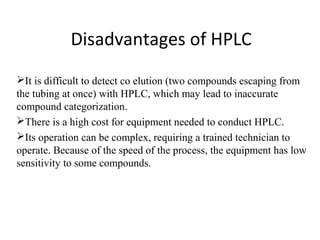
Chromatography is a technique used to separate mixtures by distributing compounds between a stationary and mobile phase. High-performance liquid chromatography (HPLC) is commonly used and separates compounds using a column with a stationary phase and liquid mobile phase. HPLC can identify, detect, quantify, and purify individual components in a mixture using an apparatus including a pump, injector, column, detector, and recorder. The separation occurs as the compounds interact differently with the stationary phase in the column.