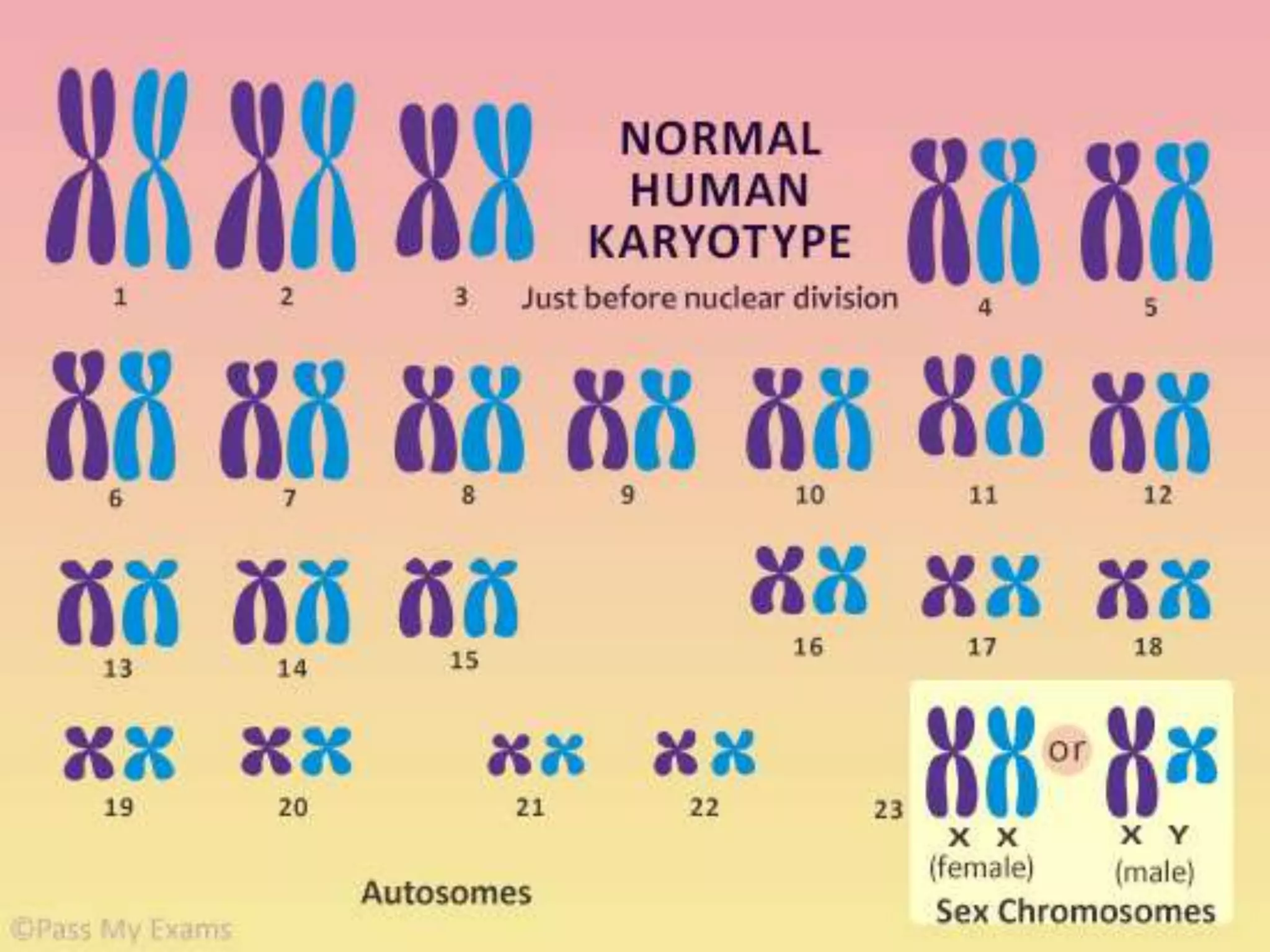
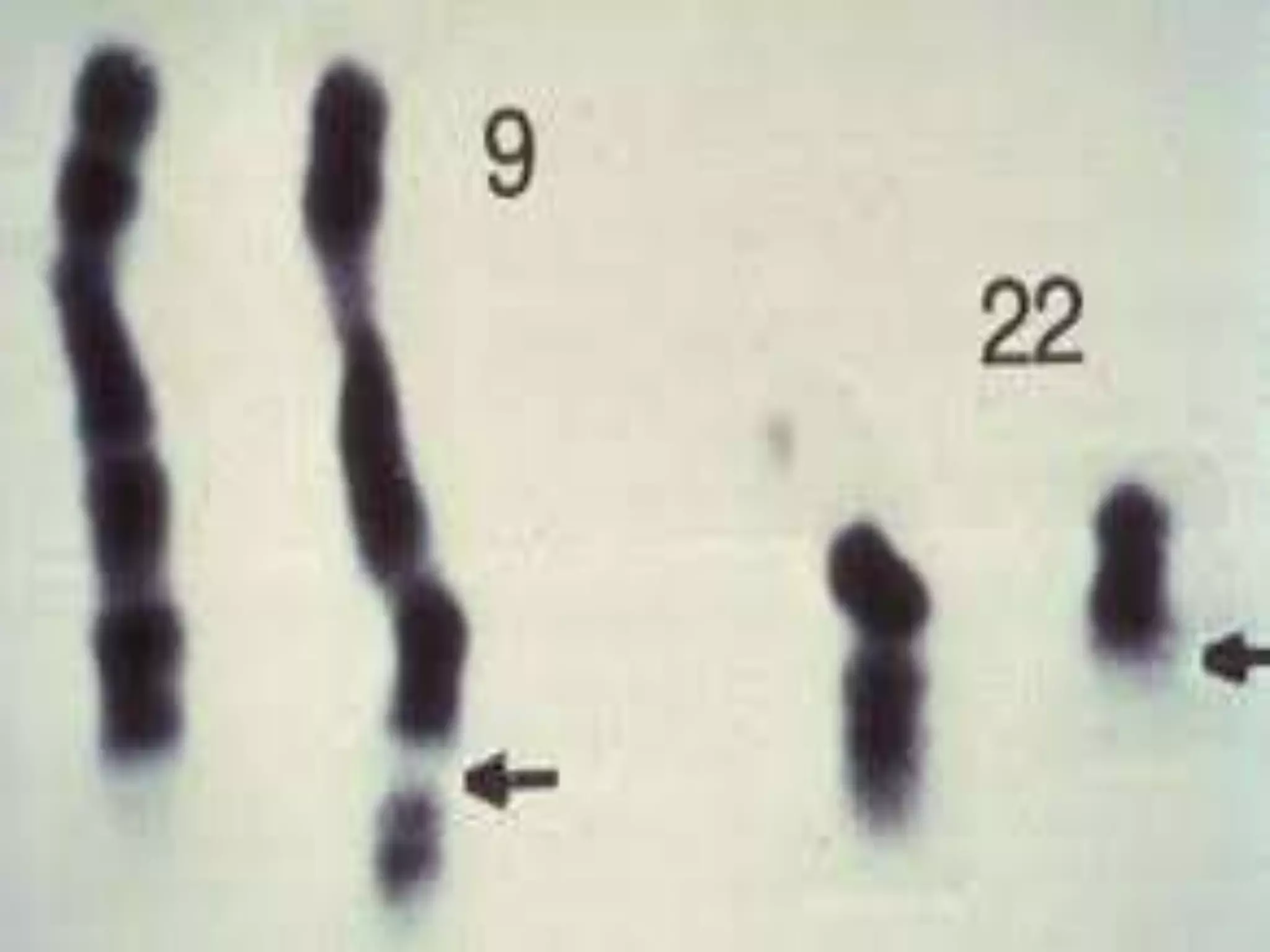
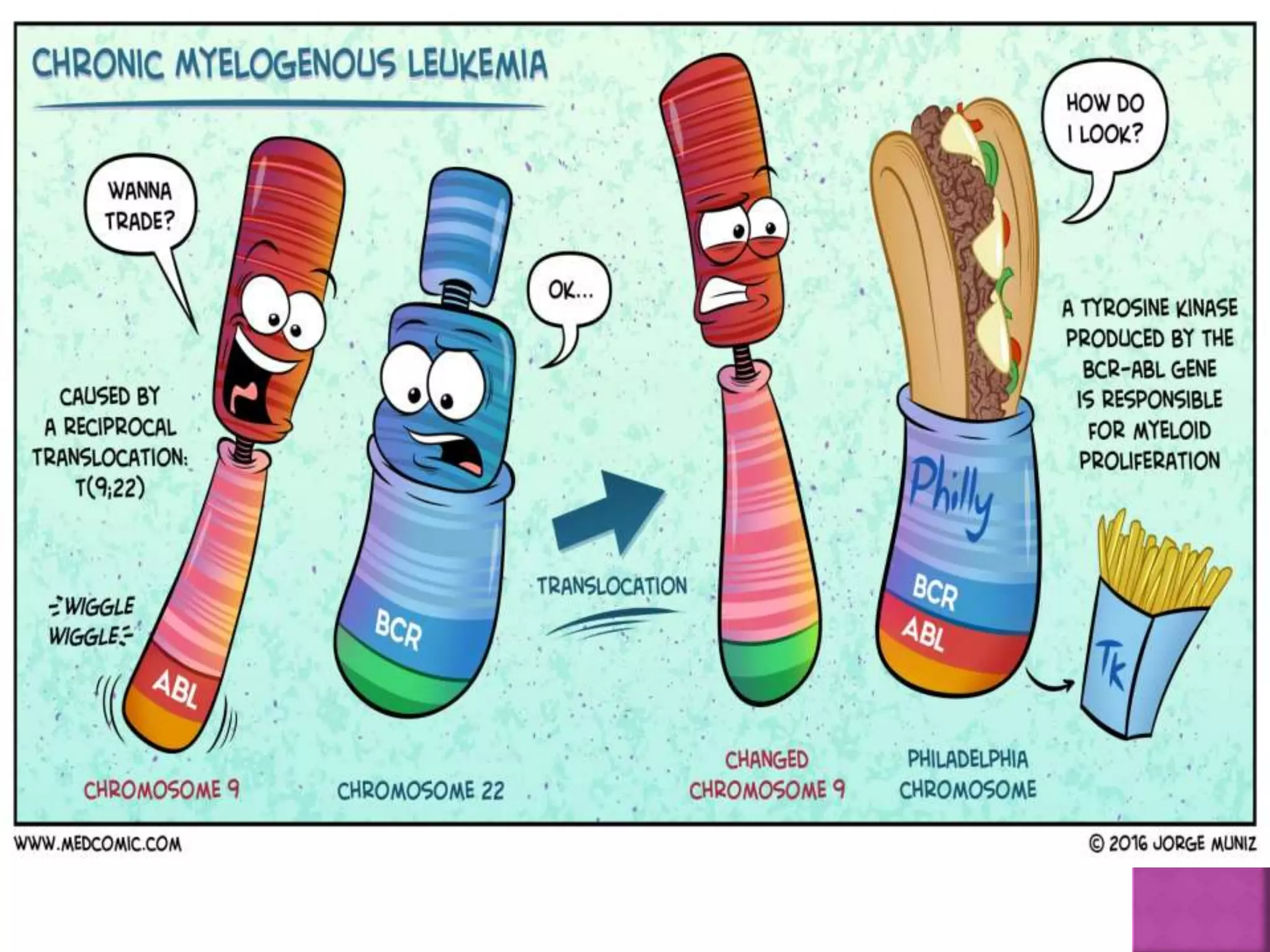
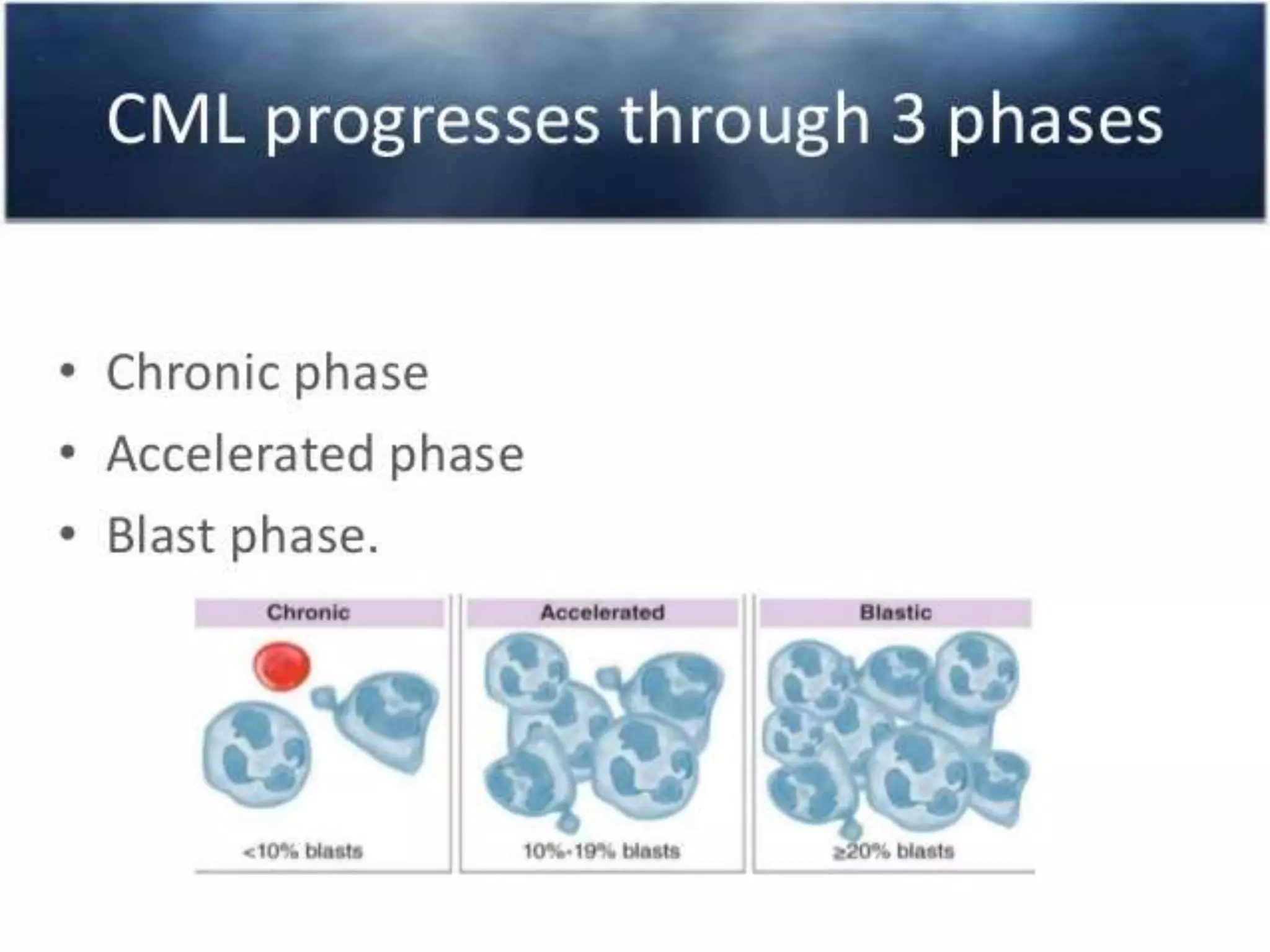
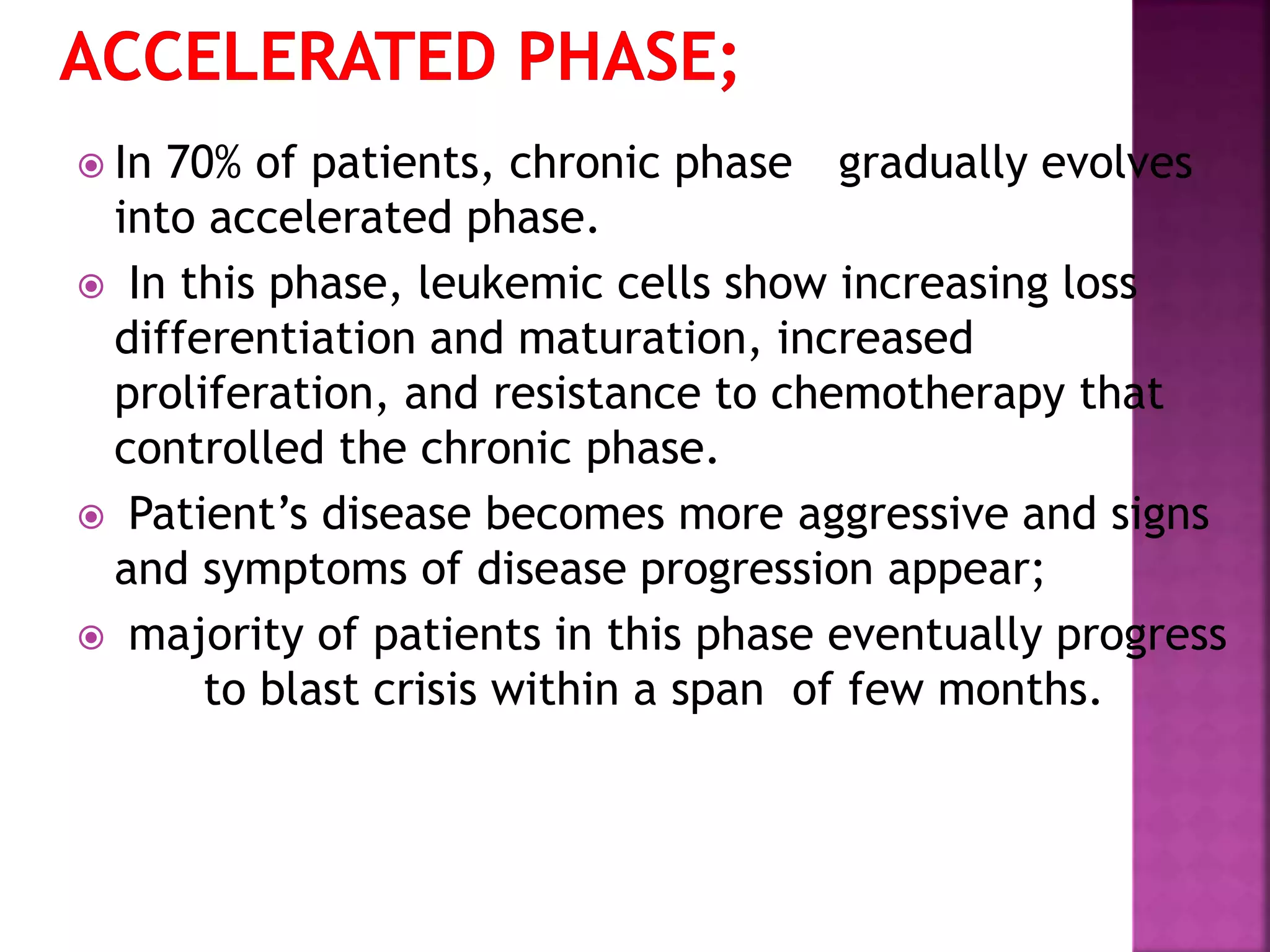
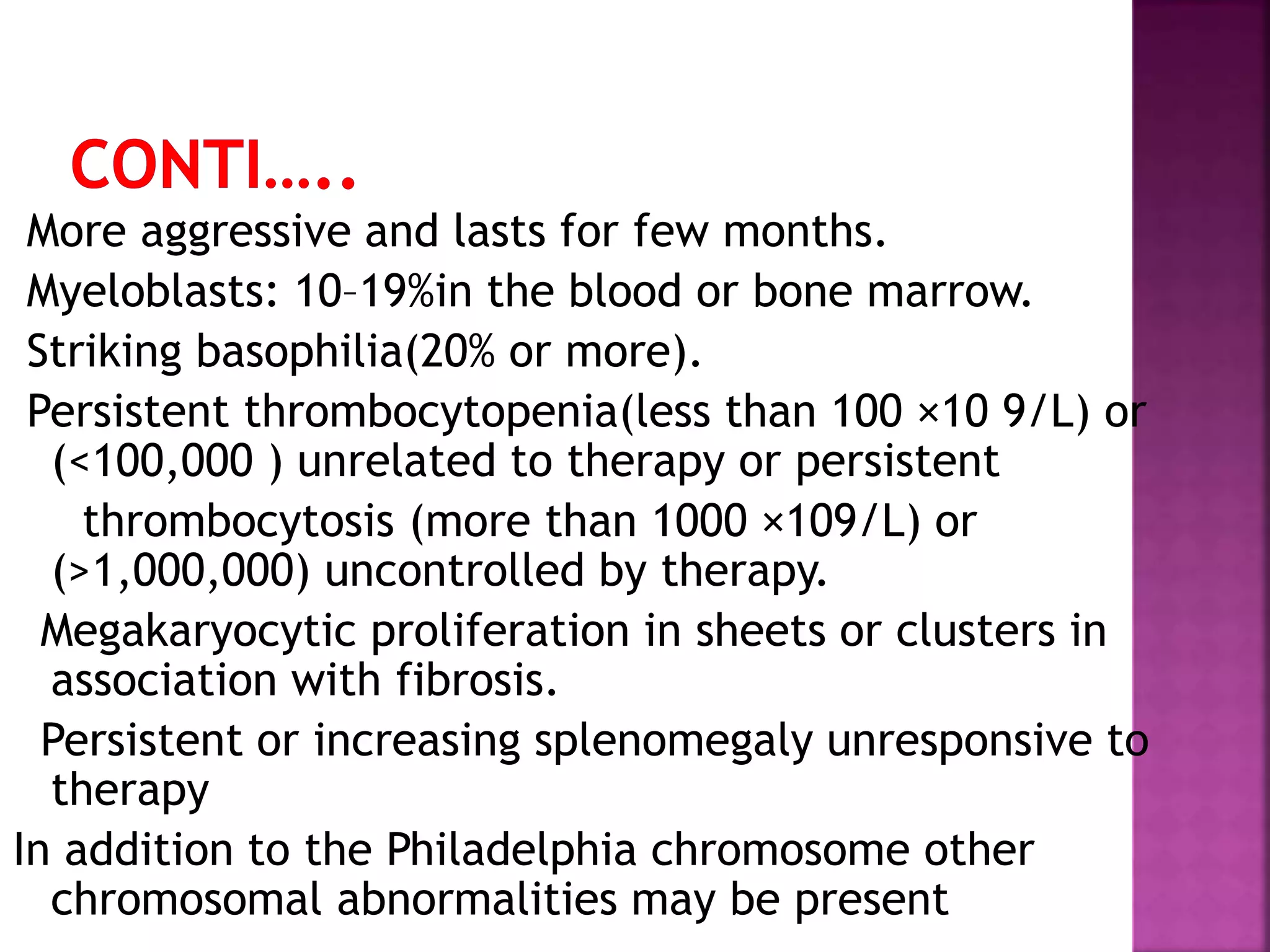
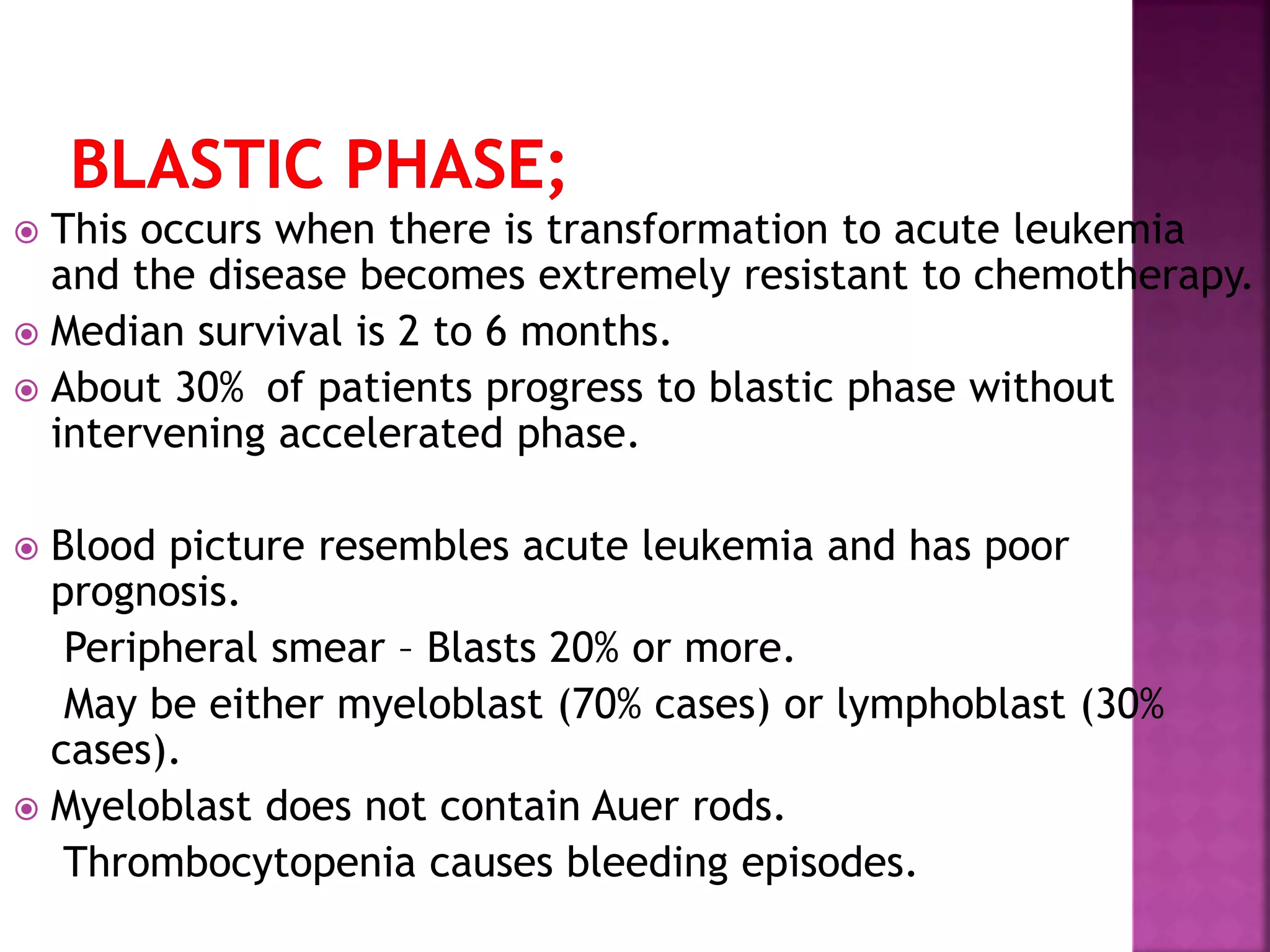
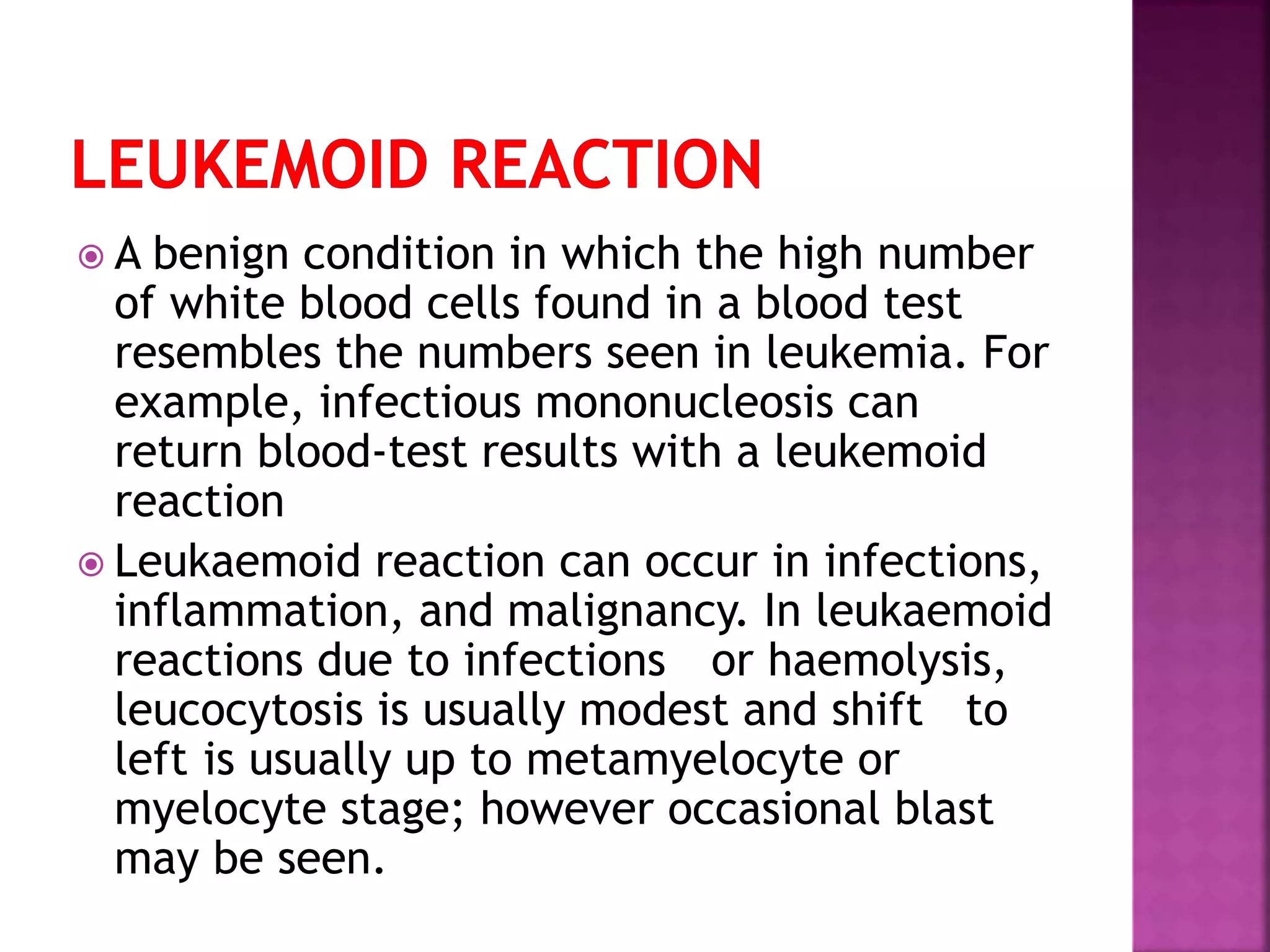
This presentation provides information about chronic myeloid leukemia (CML). It begins by outlining 8 learning objectives about CML, including defining it, describing its subtypes and epidemiology, explaining its pathophysiology and genetic alterations, comparing its clinical signs in different phases, and more. It then covers CML's introduction, pathogenesis involving the Philadelphia chromosome and BCR-ABL fusion gene, epidemiology and risk factors, clinical features in chronic and advanced phases, diagnosis methods including cytogenetics, and reference books for further information. The goal is for students to understand CML and be able to answer questions about classifying, detecting, diagnosing, and comparing its various aspects.