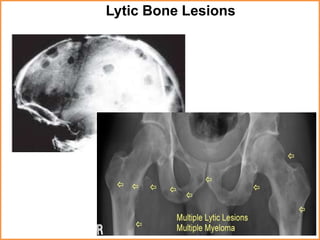
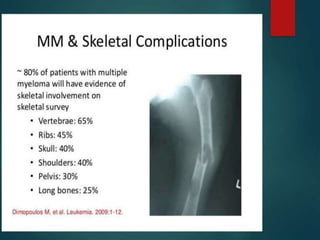
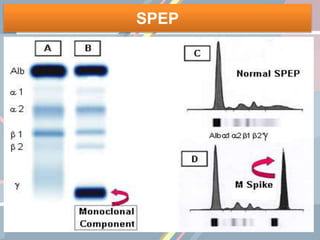
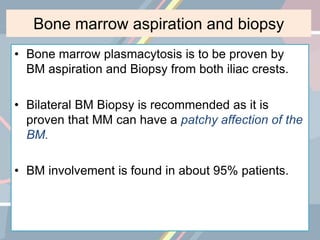
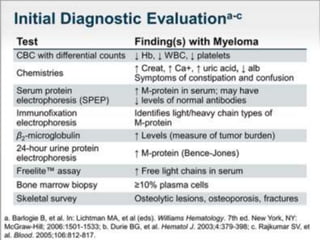
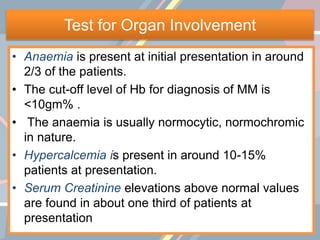
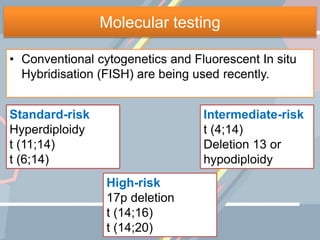
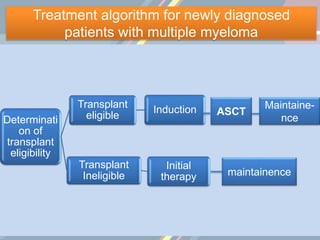
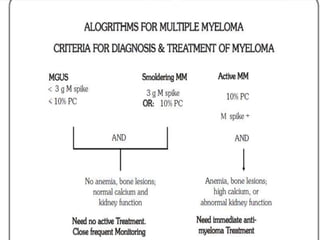
A 55-year-old male presented with new symptoms of exertional fatigue. Bloodwork found low hemoglobin and a serum protein electrophoresis demonstrated a monoclonal IgA protein. A skeletal survey showed lytic bone lesions in the skull and humeri. A bone marrow biopsy showed 30% involvement by abnormal plasma cells positive for CD138. This suggests a diagnosis of multiple myeloma based on the presence of a monoclonal protein, bone lesions, and bone marrow involvement by plasma cells.



































































































![Diarrhoea
• Antidiarrhoeals- [loperamide(Imodium),
diphenoxylate (Lomotil)]
• Antisecretory drugs - Inj.Octreotide
• Probiotics and yoghurt to maintain the natural gut
flora
• Increase fluid intake
• Low-residue, low-fat diet
• Assess daily weight](https://image.slidesharecdn.com/multiplemyeloma-200627064825/85/Multiple-myeloma-126-320.jpg)