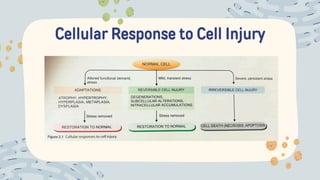
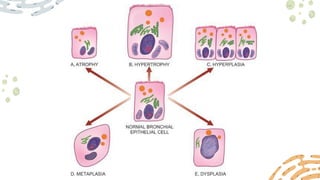
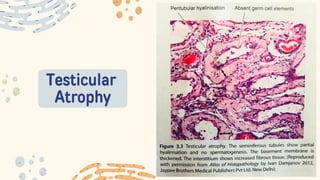
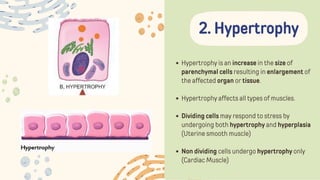
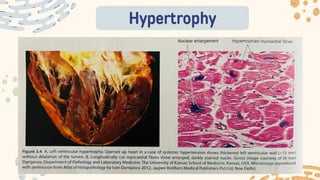
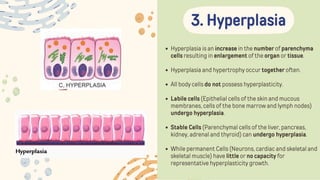
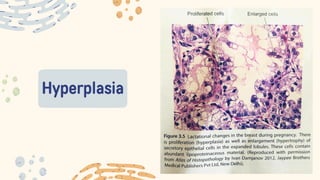
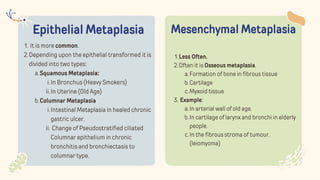
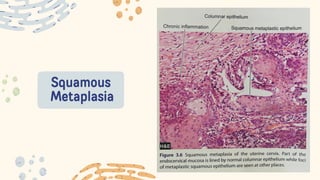
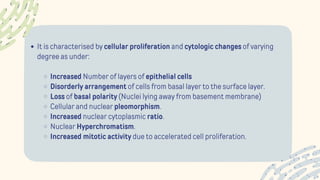
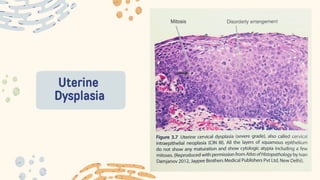
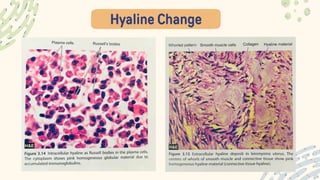
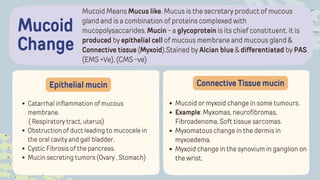
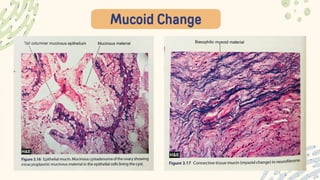
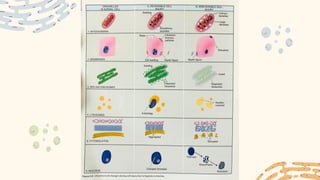
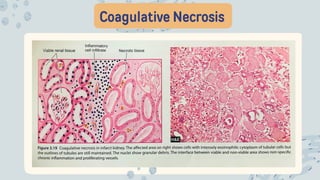
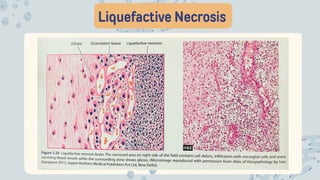
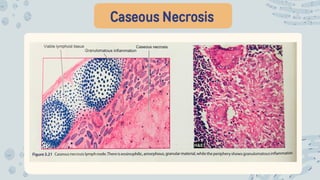
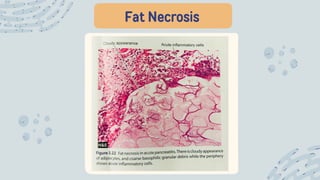
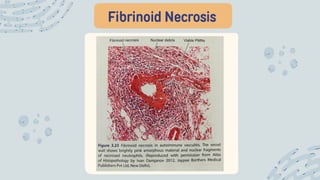
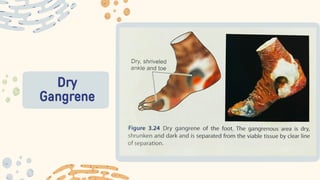
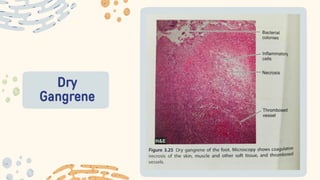
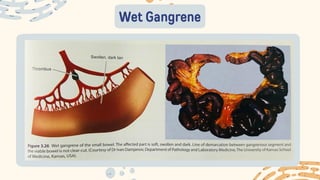
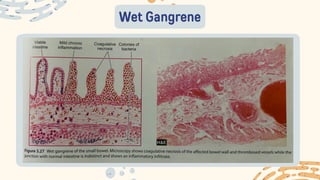
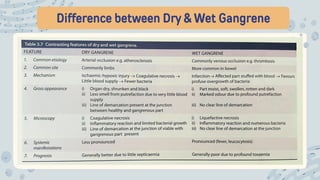
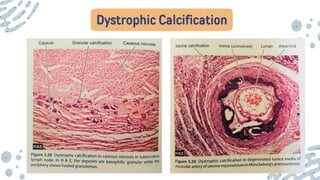
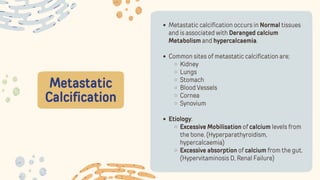
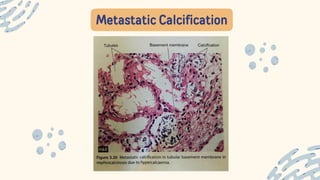
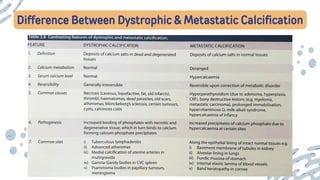
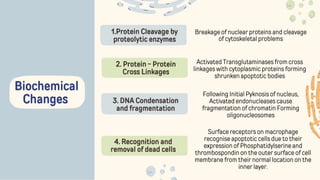
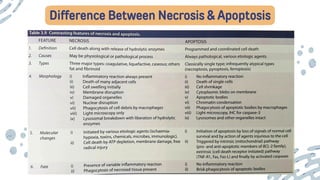
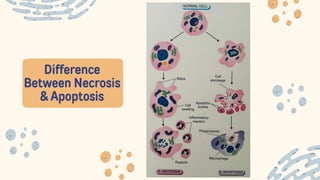
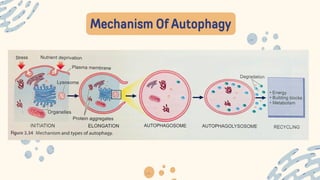
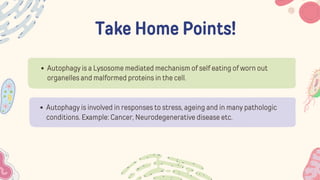
The document discusses cellular adaptations and cell injury, exploring how cells respond to various stressors such as hypoxia, physical agents, chemicals, and microbial influences. It details types of cell injury including atrophy, hypertrophy, hyperplasia, metaplasia, and dysplasia, along with distinct forms of irreversible cell injury like necrosis and apoptosis. Key points emphasize the importance of understanding cellular responses to stress and the potential for reversible injury depending on the type and duration of the stressor.