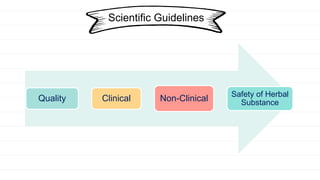
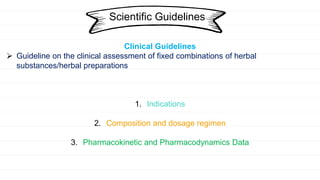
The European Medicines Agency and regulatory authorities in the EU prepare scientific guidelines to help applicants for herbal medicine marketing authorization. The guidelines provide a harmonized approach across EU states for demonstrating quality, safety, and efficacy of herbal medicines. They include guidelines on good practices for herb cultivation and collection, quality control of herbal substances and preparations, assessing genotoxicity and clinical safety/efficacy of herbal medicines, and public statements on allergic risks and contamination issues.