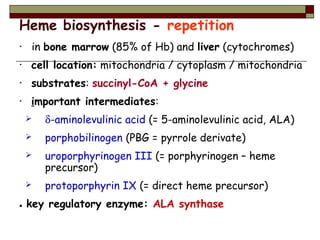
The document discusses enzymes and porphyrias. It begins by defining enzymes as proteins with catalytic properties. It then provides objectives about understanding heme structure, identifying rate-limiting steps and effects of drugs in heme biosynthesis. The rest of the document details heme synthesis, the pathway of porphyrin synthesis, different types of porphyrias caused by deficiencies in specific enzymes in this pathway, and their associated symptoms. It highlights that porphyrias can be acquired from lead toxicity or inherited congenital disorders.





![Structure of heme prosthetic group
Protoporphyrin ring w/ iron =Protoporphyrin ring w/ iron =
hemeheme
Four Pyrrole groups [A to D]Four Pyrrole groups [A to D]
linked by methane bridgeslinked by methane bridges
FeFe+2+2
coordinated by prophyrin Ncoordinated by prophyrin N
atoms and a N from Histidineatoms and a N from Histidine
(blue)(blue)
A molecule of OA molecule of O22 acts as 6acts as 6thth
ligandligand](https://image.slidesharecdn.com/26porphyria-150903110923-lva1-app6892/85/26-porphyria-6-320.jpg)