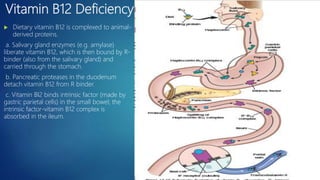
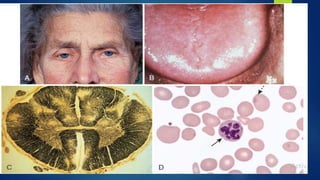
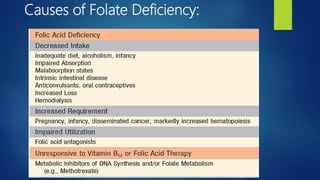
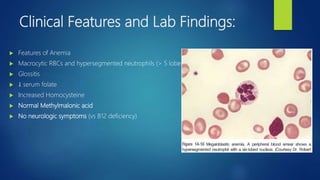
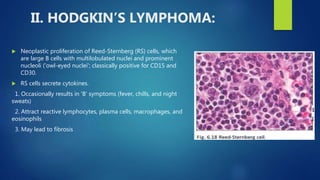
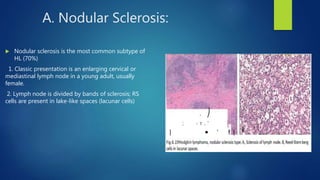
This document provides an overview of several topics in hematology, including:
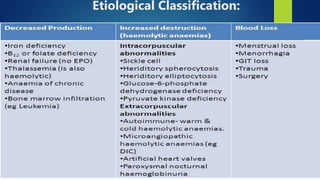
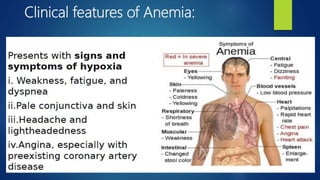
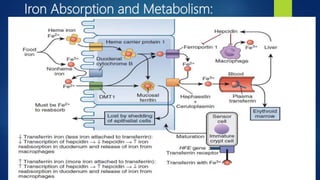
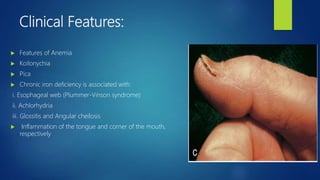
- Anemia, which is a reduction in red blood cells. Common types are discussed such as iron deficiency anemia and megaloblastic anemia.
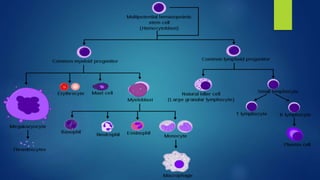
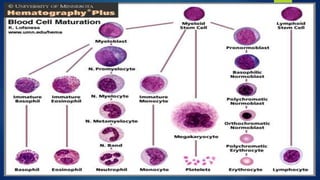
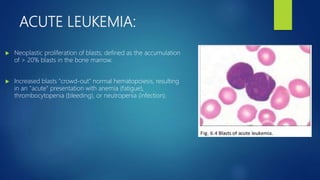
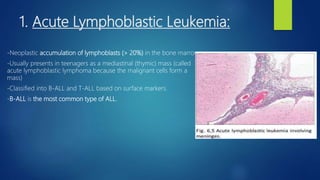
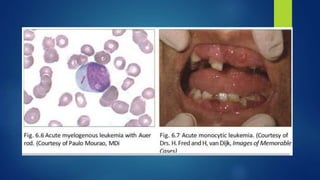
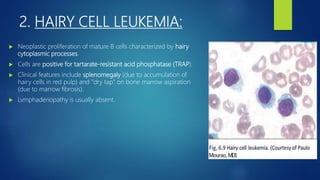
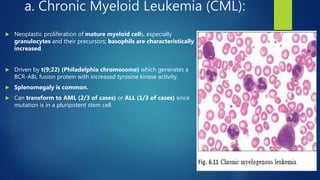
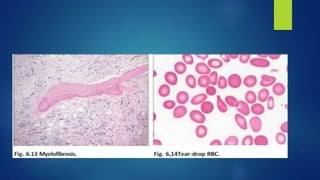
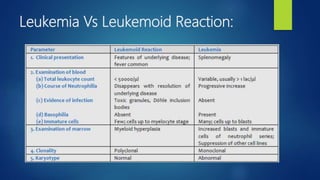
- Leukemia, which are cancers of the white blood cells. The main types - acute and chronic leukemias - are defined. Acute leukemias like ALL and AML are discussed in more detail.
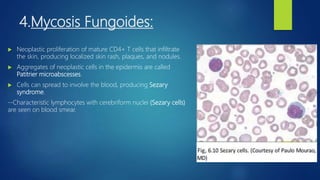
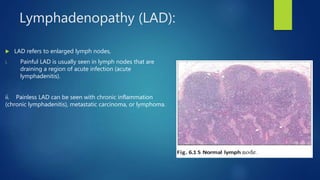
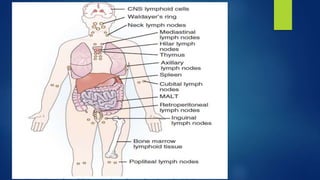
- Other blood disorders like lymphomas and lymphadenopathy are listed as learning objectives but not described further in this document.