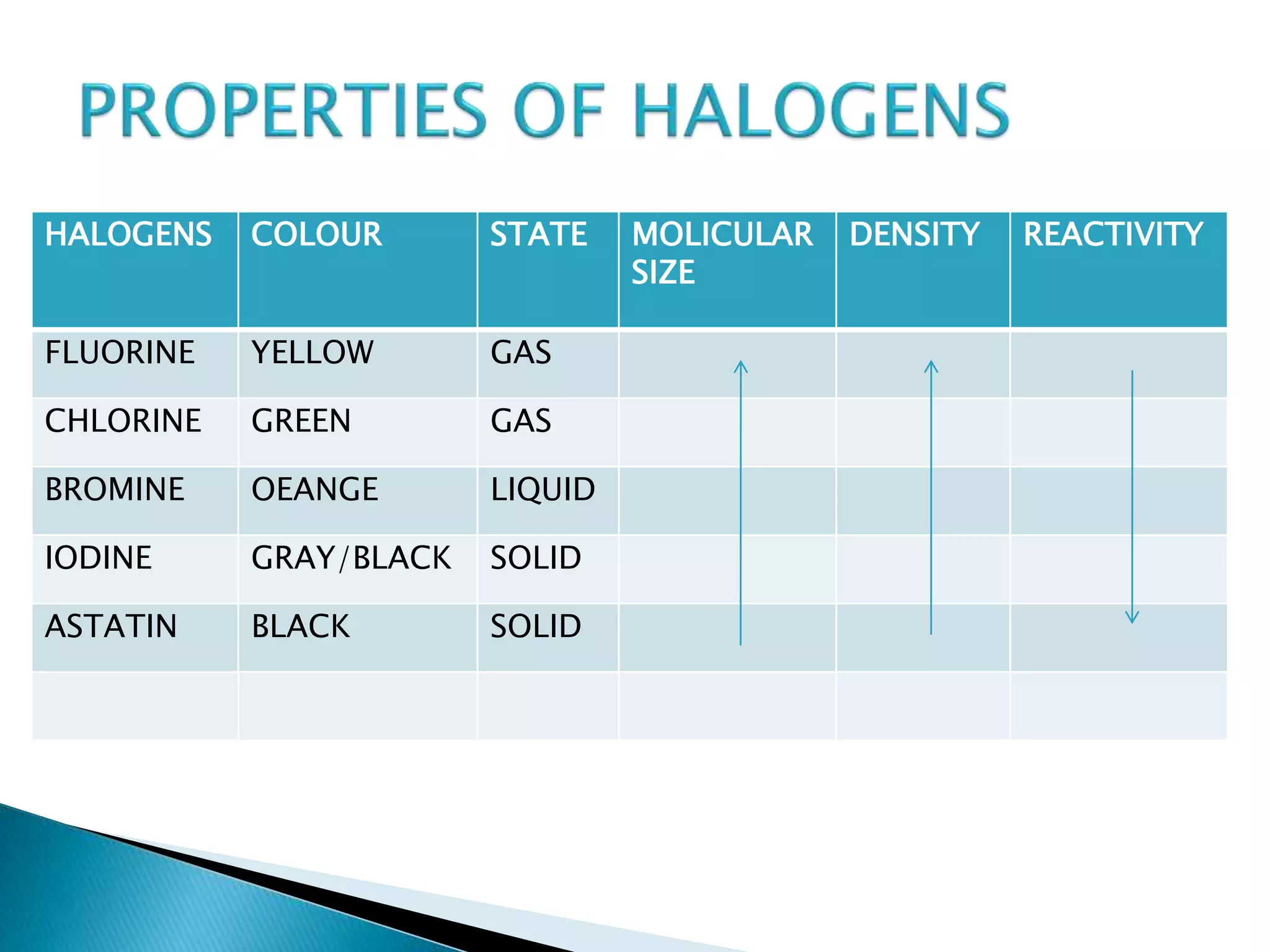
Halogenated compounds contain one or more halogens, which include fluorine, chlorine, bromine, iodine, and astatine. Halogens are highly reactive due to their high electronegativity. Halogenated compounds have many industrial uses such as solvents, paints, plastics, and pharmaceuticals. However, they can also be toxic to humans depending on the type of compound, exposure, and concentration. Proper safety precautions and equipment are needed when working with halogenated compounds.