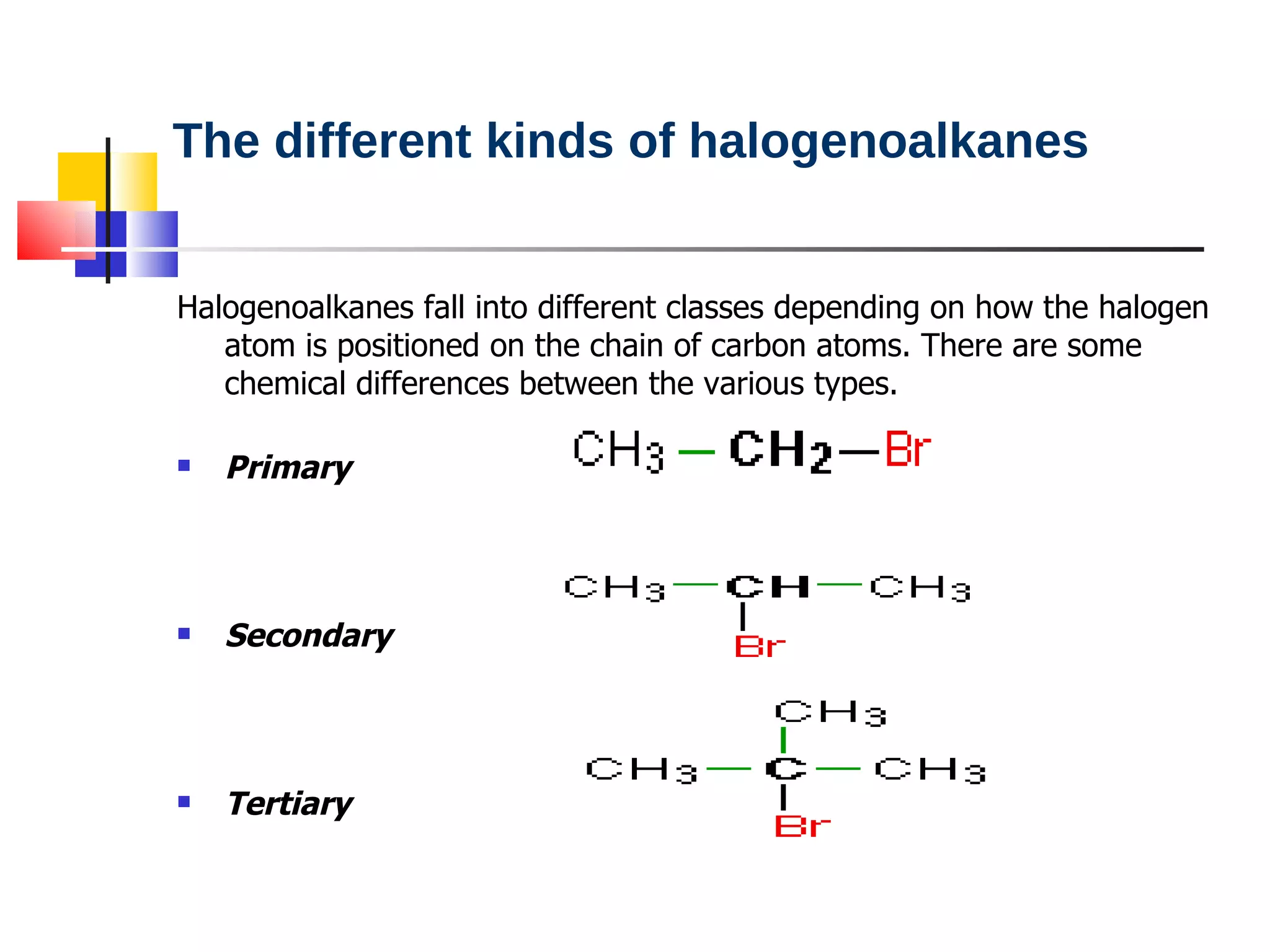
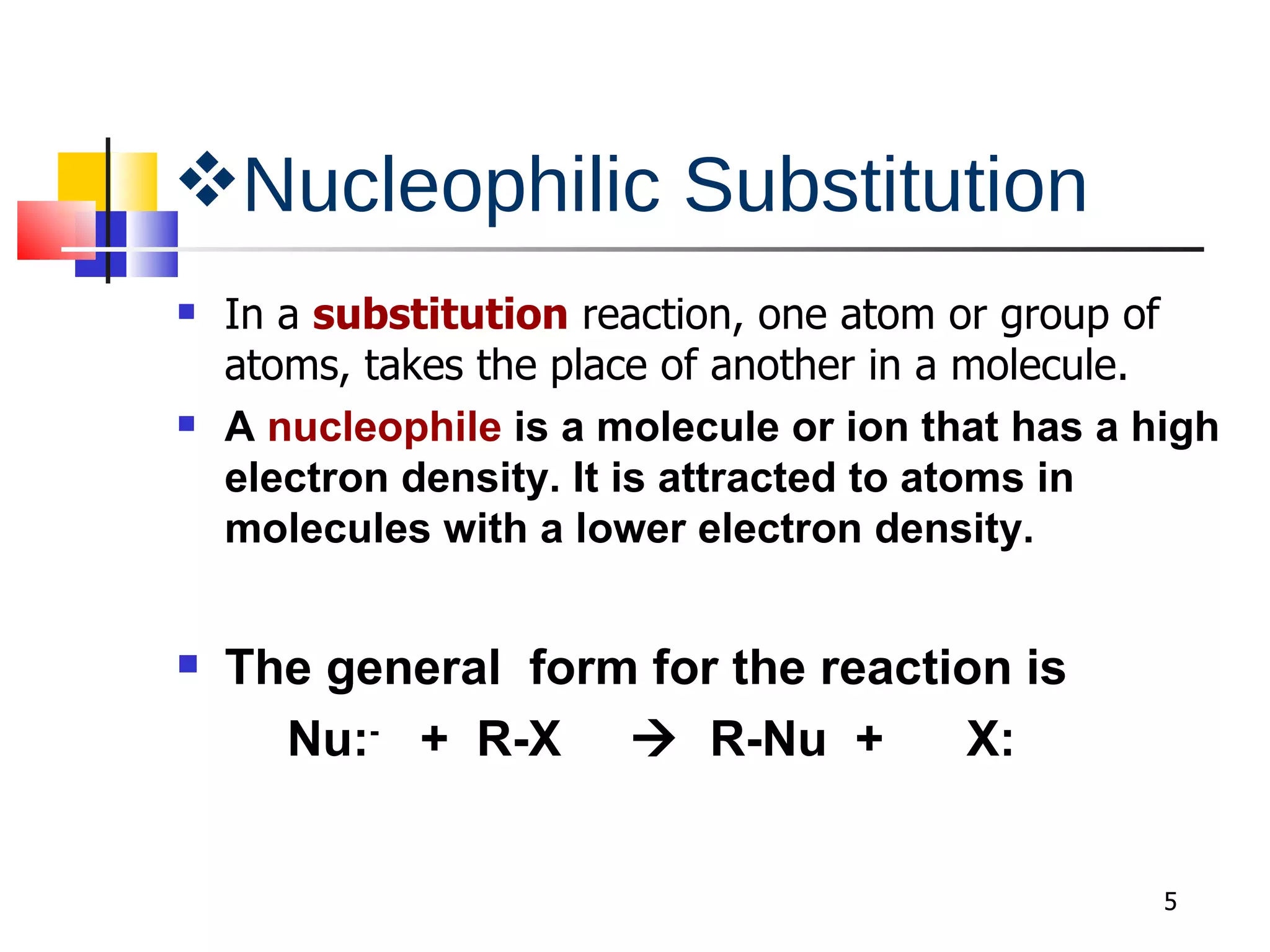
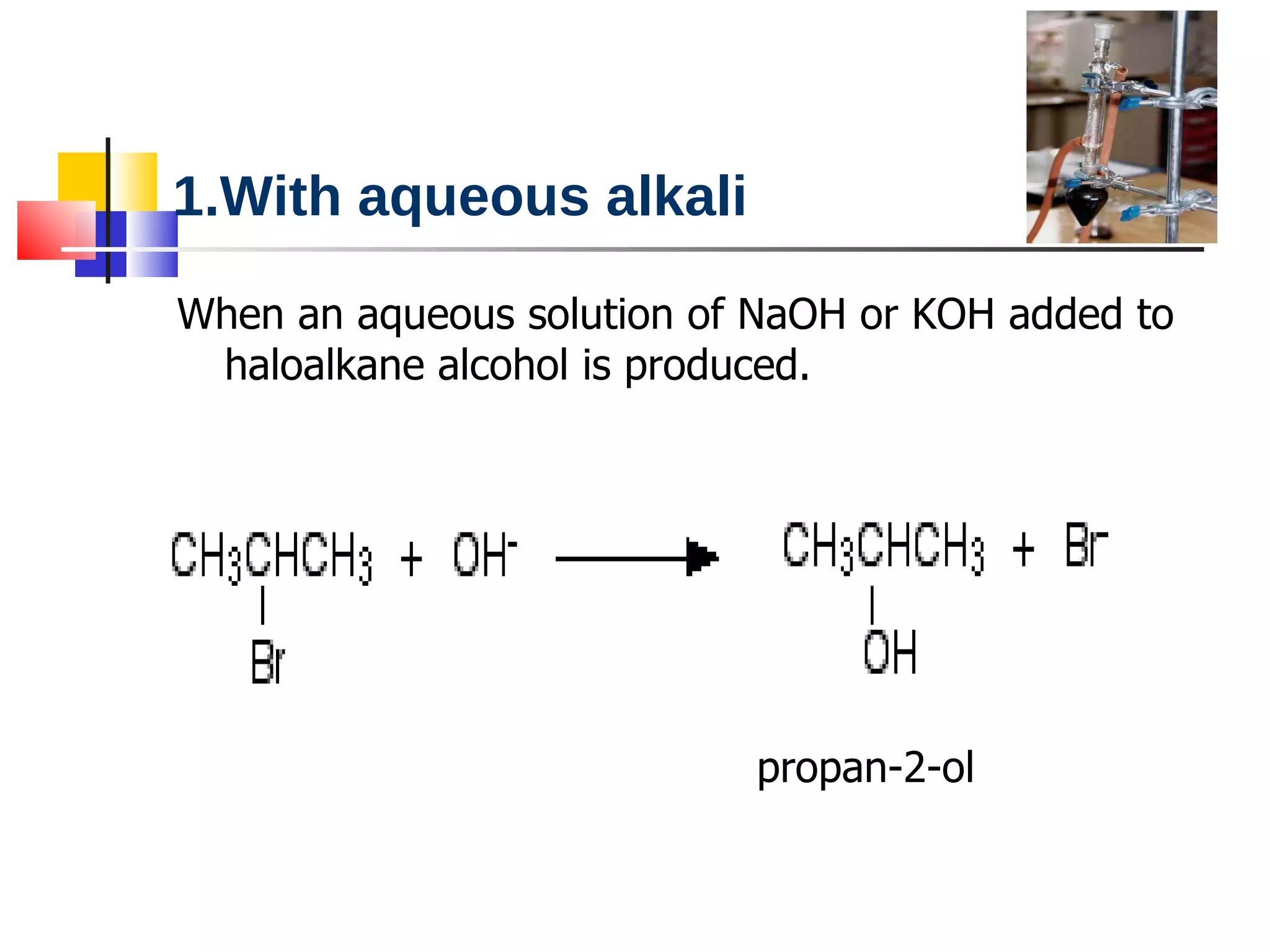
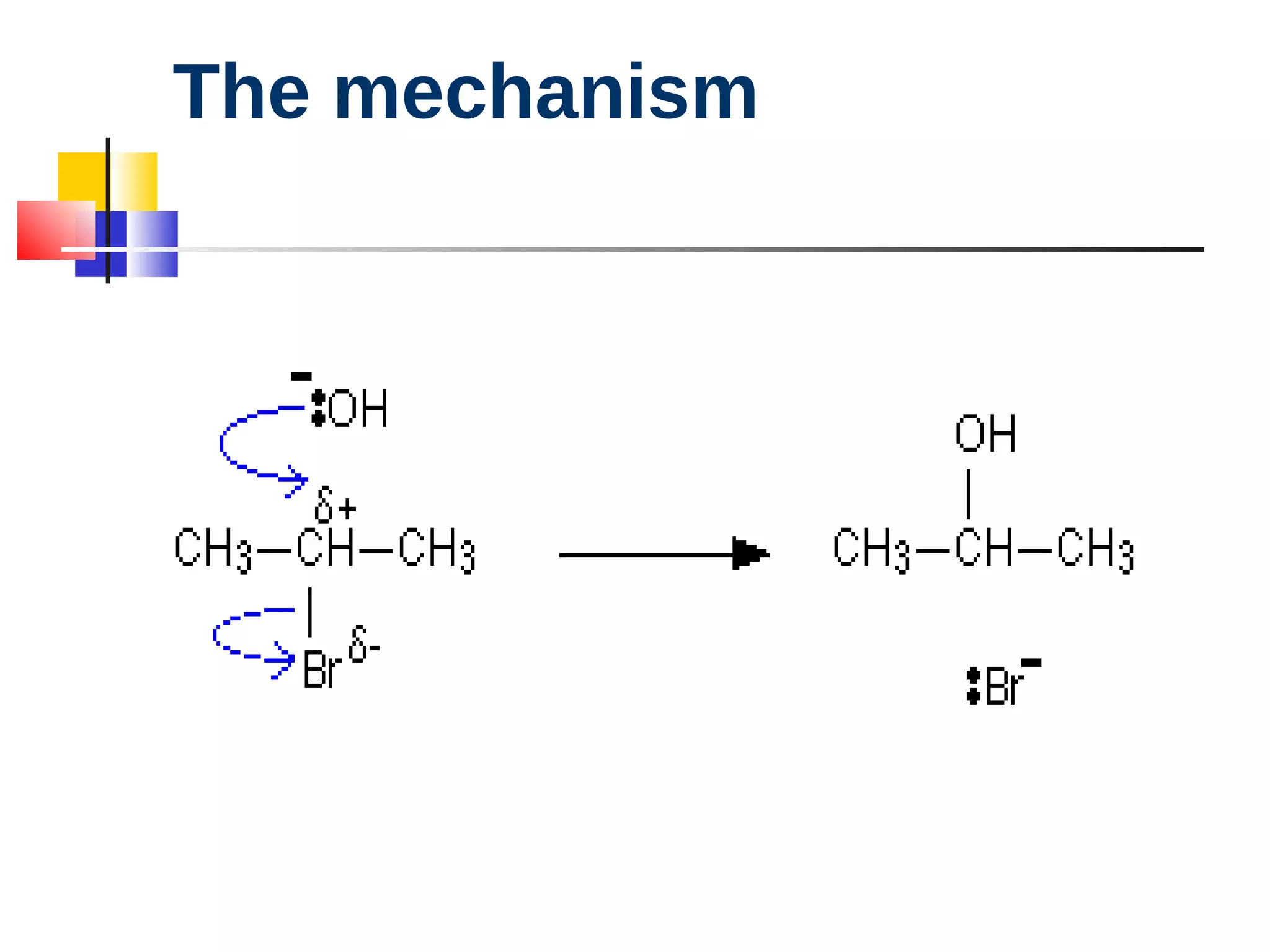
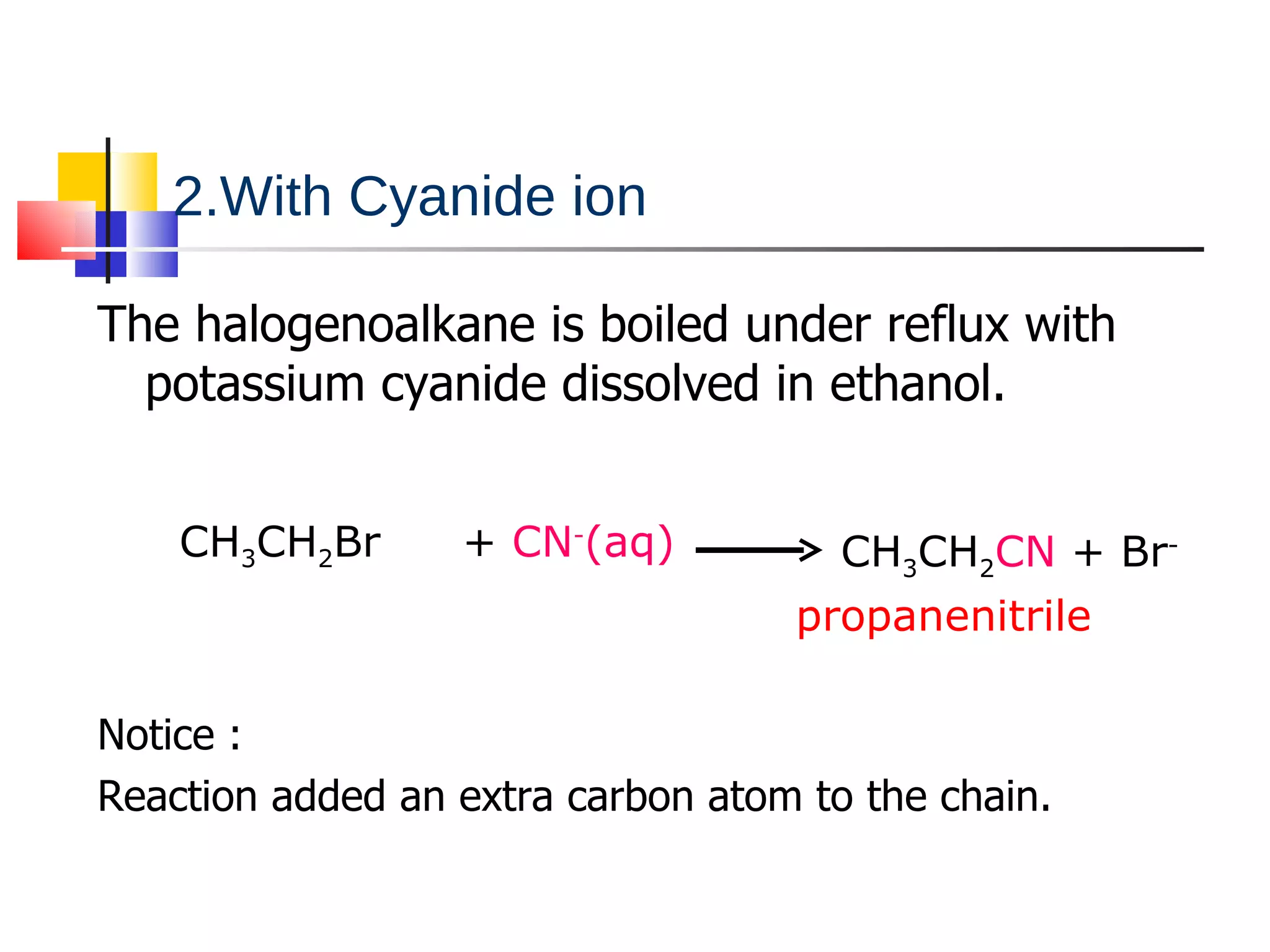
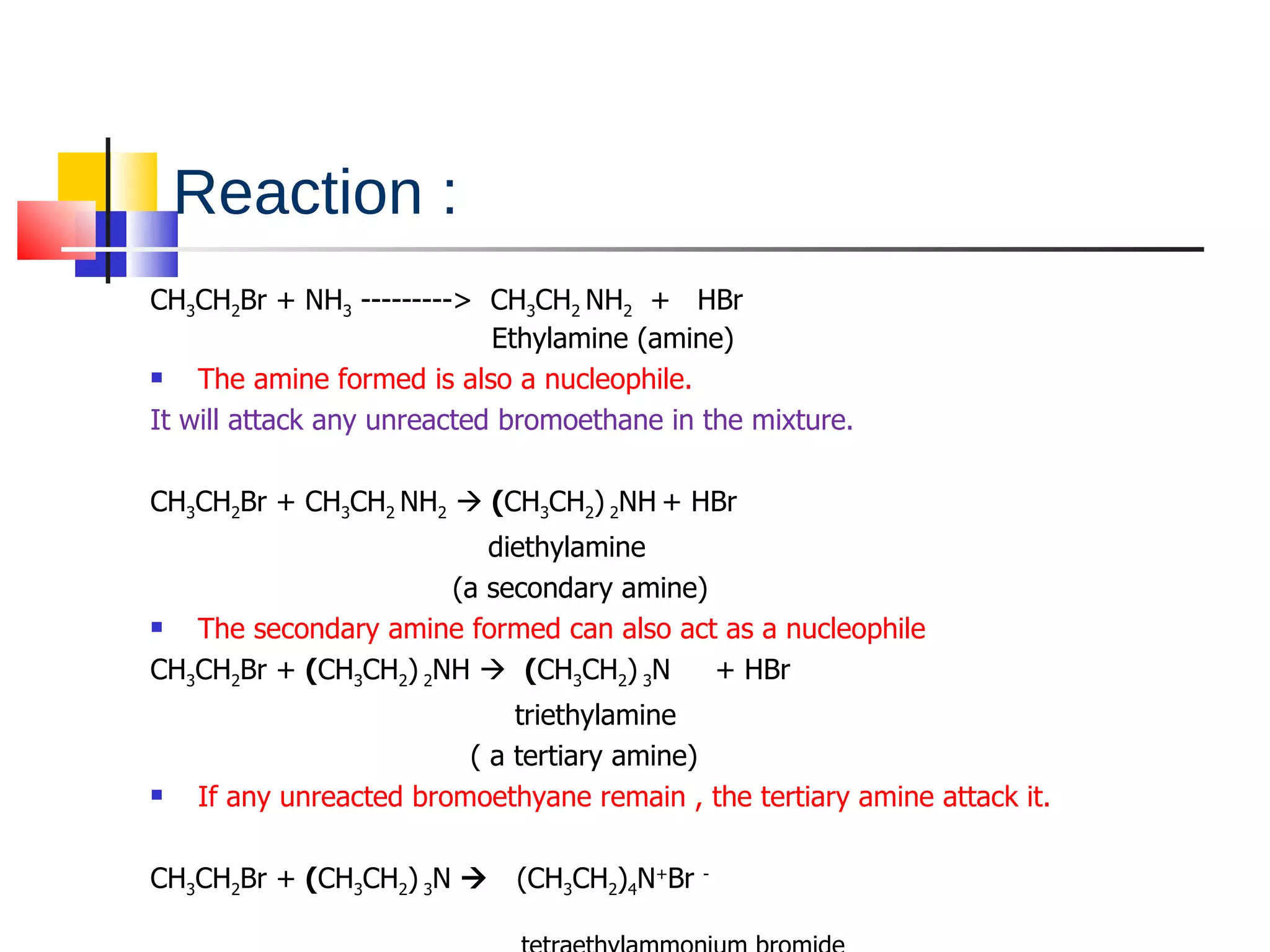
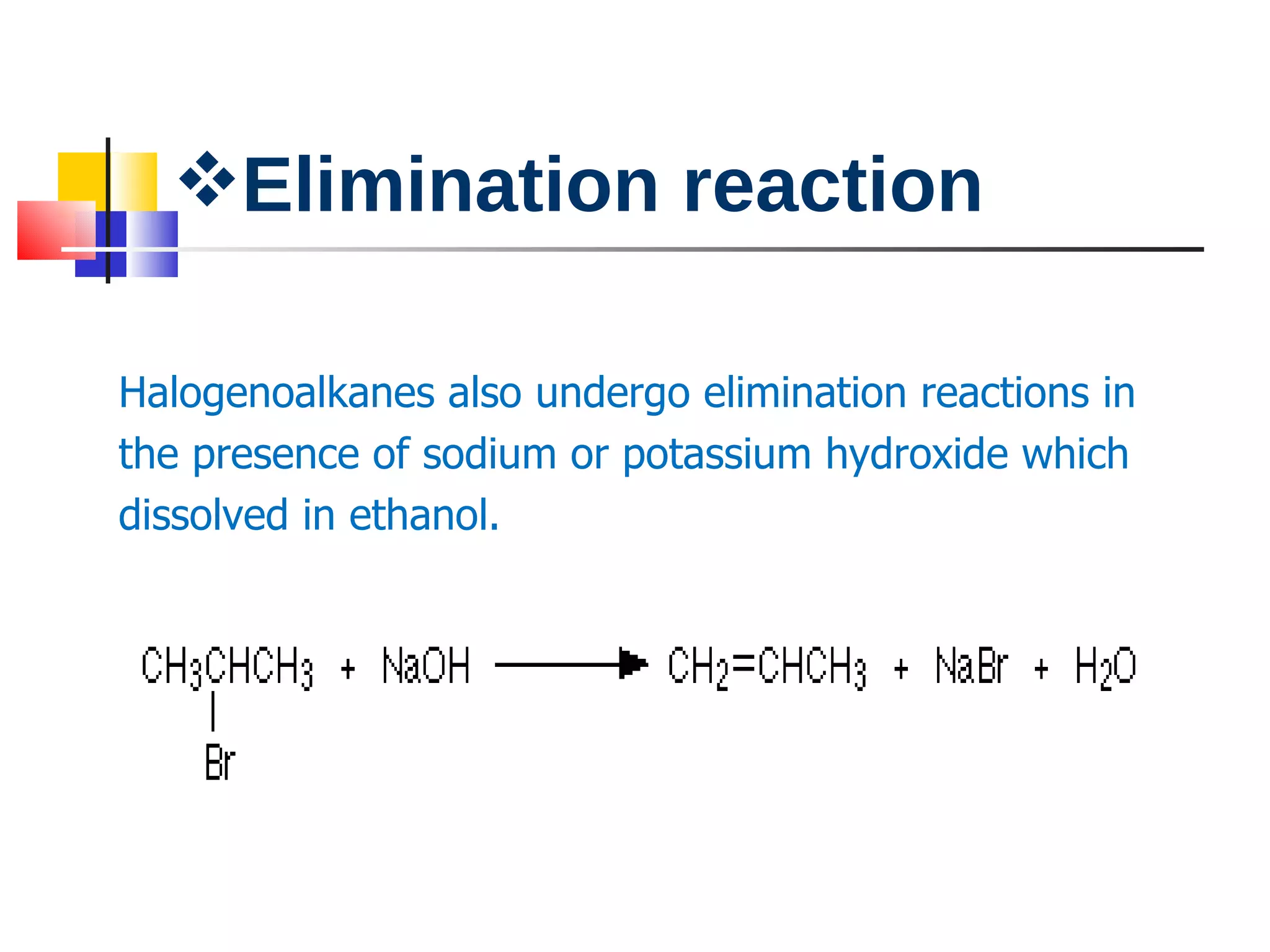
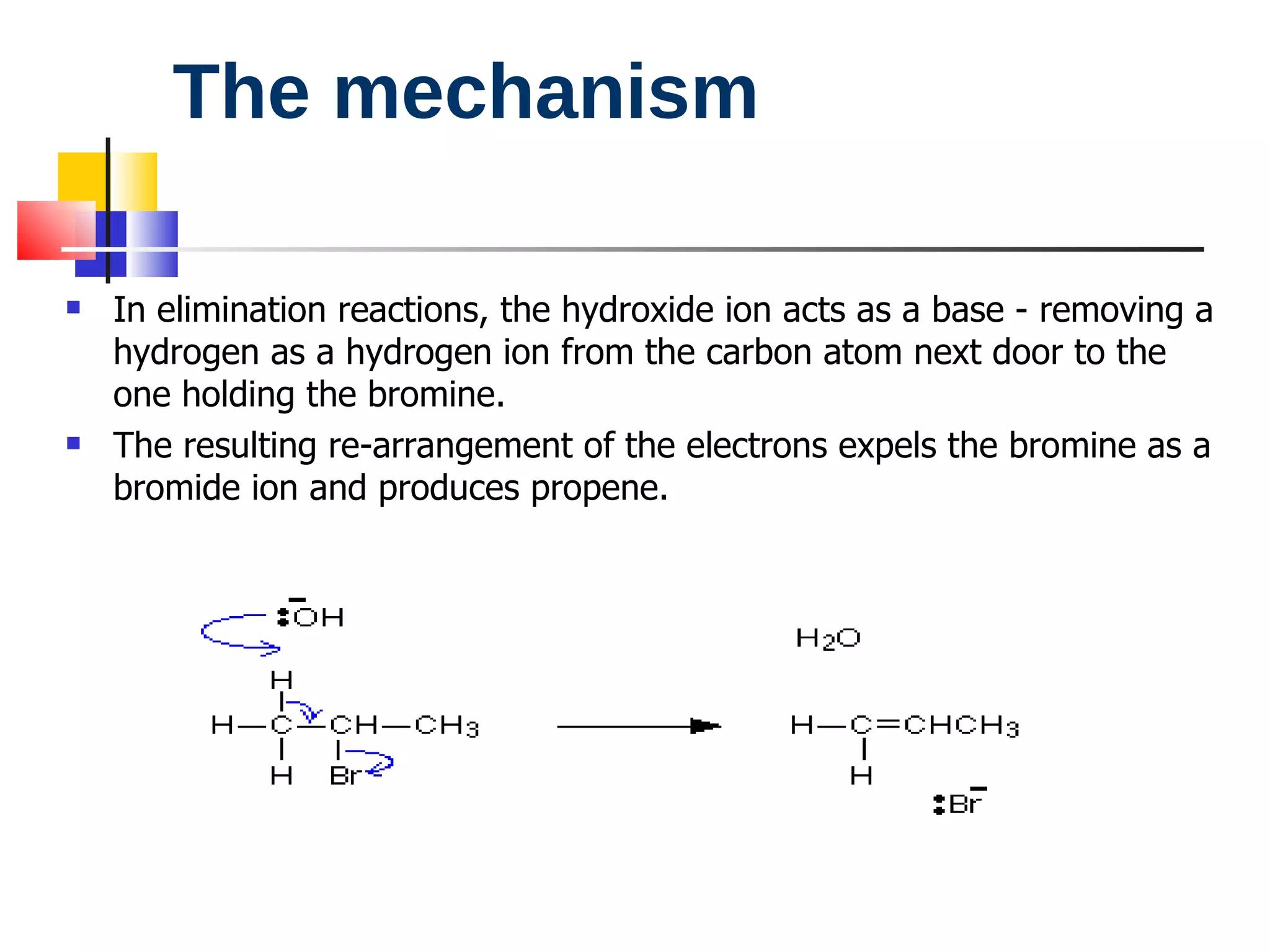
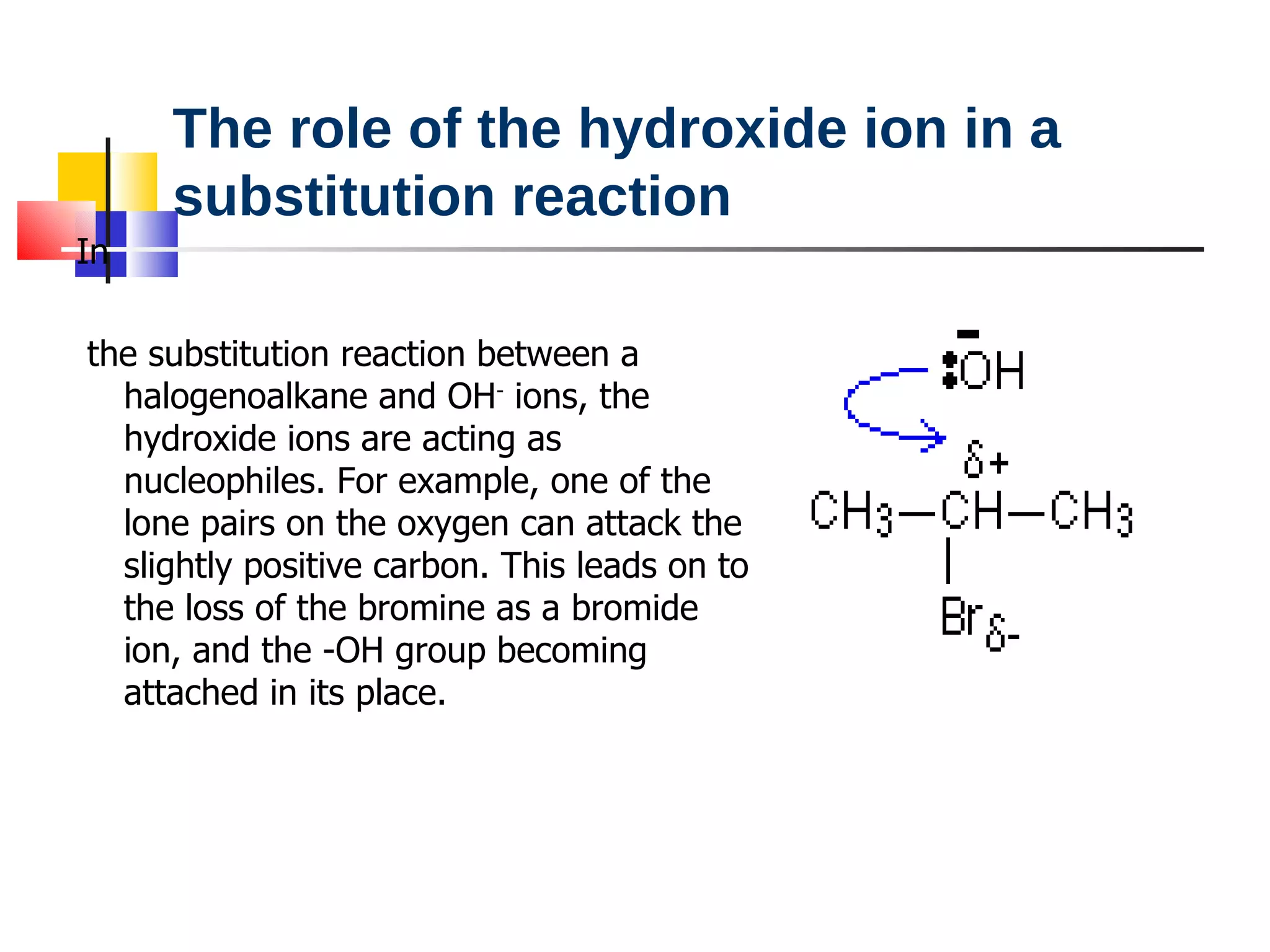
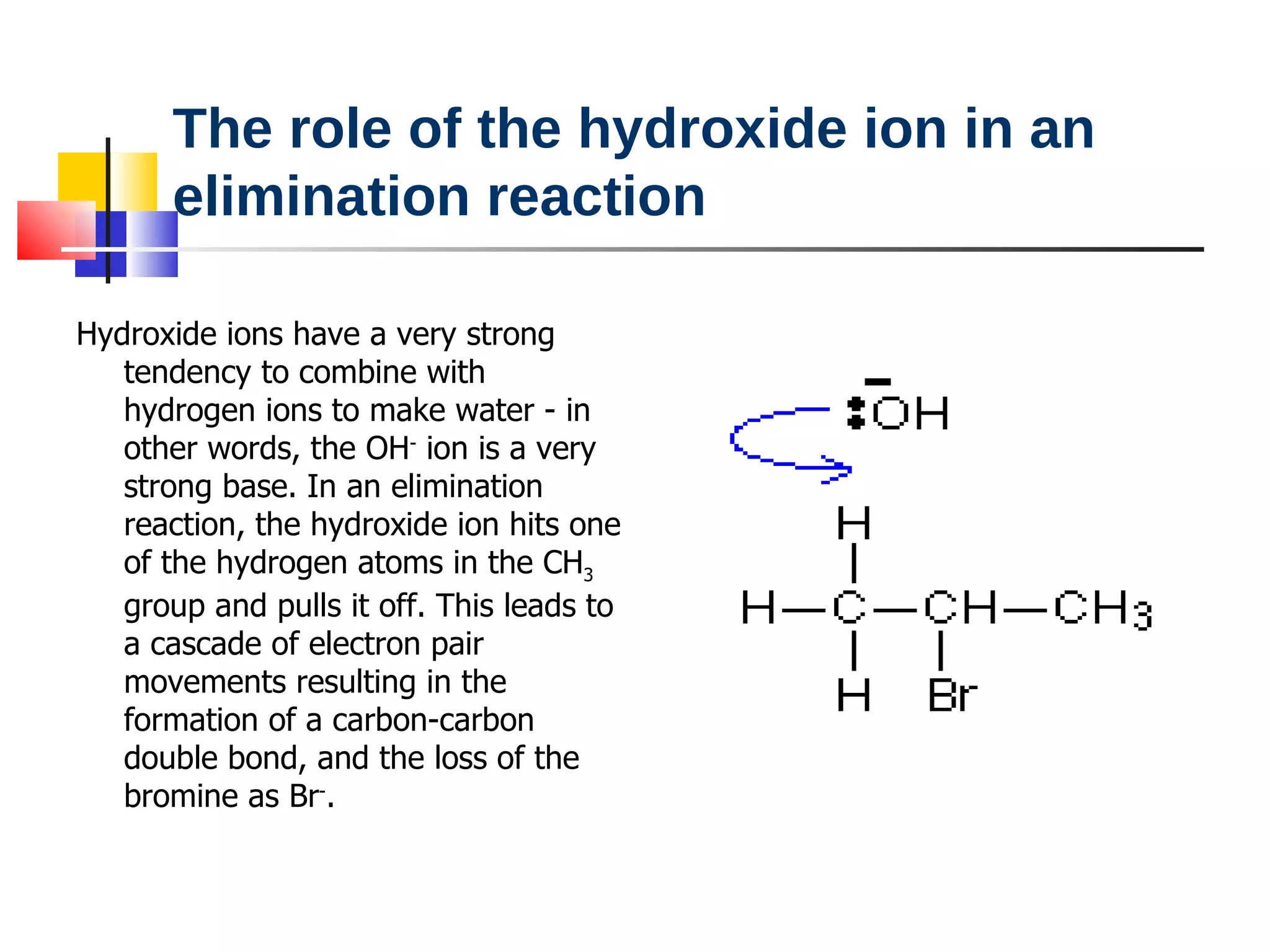
This document summarizes halogenoalkanes, which are hydrocarbons where one or more hydrogen atoms are replaced by halogen atoms. It describes the different types of halogenoalkanes based on the position of the halogen. The document also summarizes two main reactions of halogenoalkanes: nucleophilic substitution and elimination. In nucleophilic substitution, a nucleophile such as hydroxide replaces the halogen. In elimination reactions, hydroxide removes a hydrogen next to the halogen, expelling the halogen and forming a double bond.