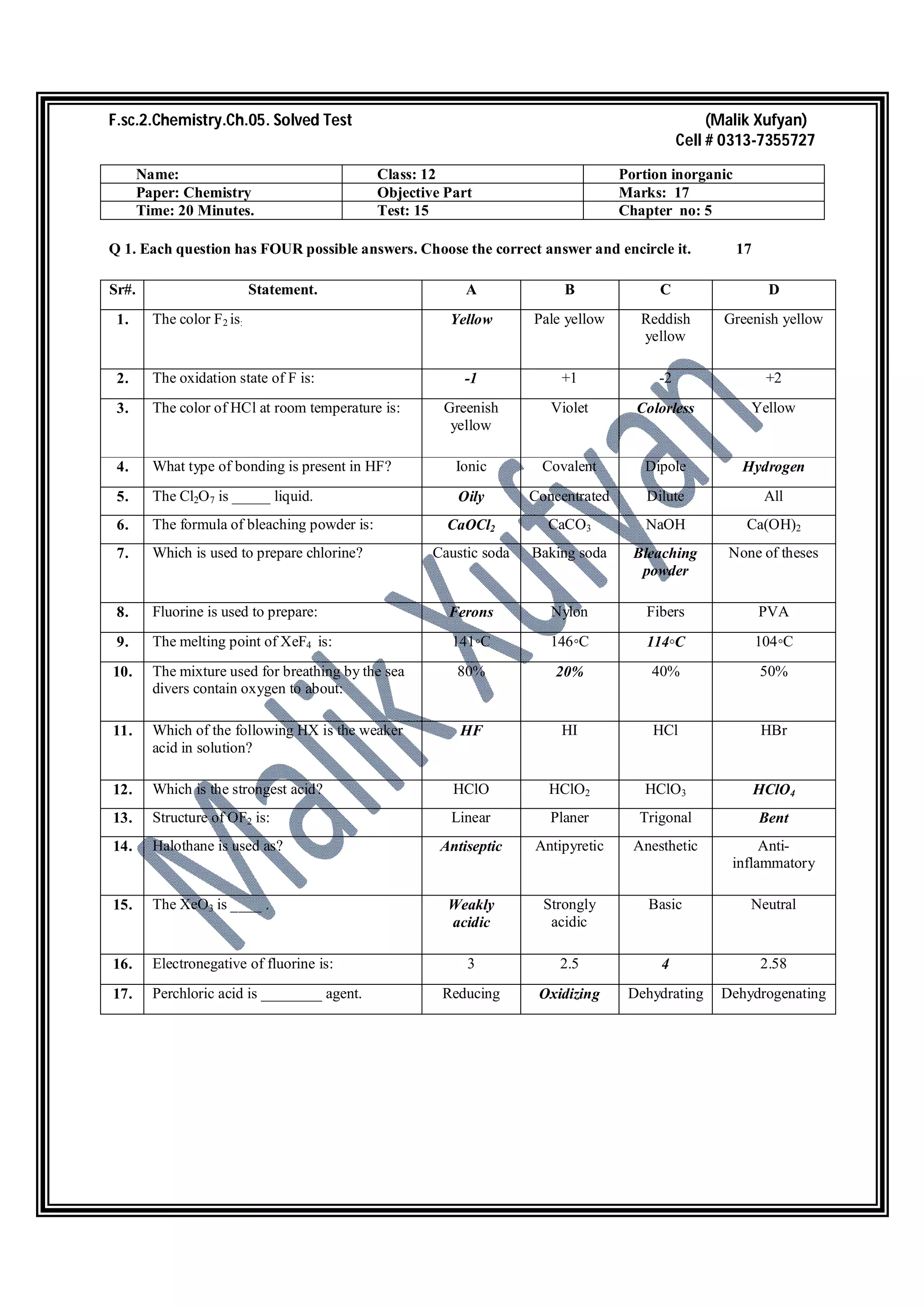
1. The document contains questions from a chemistry test on Chapter 5 covering topics like halogens, noble gases, oxides and compounds.
2. Short answers are provided for questions asking about bleaching powder preparation, disproportionation reactions, iodized salt, uses of halogens, noble gas properties and more.
3. Longer answers discuss similarities and differences of fluorine, oxidizing properties of halogens, reactions of chlorine with sodium hydroxide, and industrial bleaching powder manufacture.

![F.sc.2.Chemistry.Ch.05. Solved Test (Malik Xufyan)
Cell # 0313-7355727
Name: Class: 12 Portion Inorganic
Paper: Chemistry Subjective Part Marks: 68
Time: 2:40 Test: 15 Chapter no: 5
SECTON –I
Q 2. Give the short answers of the any EIGHT questions.
16
xiii. What is bleaching powder? How it is prepared give names of different methods.
Chemical formula of bleaching powder is CaOCl2 or Ca( OCl )Cl. It can be prepared on
industrial scale. Different methods are used for the manufacturing of bleaching powder.
These methods are
1. Hasenclever’s method.
2. Beckmann’s method.
Hasenclever’s method is an old method while Beckmann’s method is a modern method.
xiv. Discuss the oxides of chlorine.
Oxides of chlorine are unstable. They are not prepared by the direct reaction of chlorine aand
oxygen. These are used extensively for bleaching wood, paper pulp in industry and for water
treatment. There are two oxides of chlorine. These are chlorine dioxide and chlorine
heptaoxide.ClO2 is prepared by the following reaction.
2ClO3ˉ + 2Clˉ + 4H⁺ 2ClO2 + Cl2 + 2H2O
Chlorine heptachloride is prepared by the following reaction.
2HClO4 + P2O5
-10 o
C
Cl2O7 + 2HPO3
ClO2 used as antiseptic. It is used for purification of water. it is also used to bleach cellulose
material.
xv. What are disproportion reactions? Give an example.
A reaction in which specie (atom, ion or molecule) is oxidized and reduced simultaneously is
called disproportion reaction. For example:
Reaction of Cl2 with cold and hot NaOH is an example of disproportion reaction.
xvi. What is iodized salt?
When some amount of iodide ions are added in common salt (NaCl) , this is called iodized
salt. Usually, for this purpose NaI or KI is mixed with NaCl. Insufficient amount of iodine
causes the enlargement of thyroid glands.
xvii. What are ferons and Teflon?
Flourochlorocarbons are called ferons. These are CCl2F2 (diflourodichloromethane) & CClF3
(tifluorochloromethane). These gases are used as refrigerant and aerosol poll ants. Teflon is a
polymer of ( [ CF2-CF2 ]n ). It is an important plastic.
xviii. Why iodine has metallic lustre?
Metallic luster in iodine is due to the excitation of electrons of iodine at room temperature.
Due to the bigger size of iodine the electrons of iodine at room temperature take energy and
go to higher energy states. When excited electrons come back, they emit some radiations of
particular wavelength. Therefore, they appear as luster grayish black solid.](https://image.slidesharecdn.com/fsc-200803131854/85/F-sc-2-Chemistry-Ch-05-Solved-Test-Malik-Xufyan-3-320.jpg)
![F.sc.2.Chemistry.Ch.05. Solved Test (Malik Xufyan)
Cell # 0313-7355727
xix. Give the various uses of halogens and their derivatives.
Uses of halogens or their derivatives are
Fluorine is used in the preparation of ferons and Teflon.
Fluorides in toothpastes build a protective coating on teeth.
Chlorine is used in the manufacturing of bleaching powder, PVC, CHCl3 and CCl4.
Bromine is used as fungicide and as AgBr in photography.
Iodine is used in pharmaceutical company. It is added as NaI or KI as a table salt.
xx. Name the gas, which is used for earthquake prediction.
Radon is used for earthquake prediction.
xxi. What are noble gases? Explain their inertness on the basis of their electronic
configuration.
Elements Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Ra)
that are placed in zero group or VIII group or periodic table are called noble gases. These are
also called inert gases or rare gases because they are chemically inert and present in very
small amount in atmosphere. All noble gases have their complete octet. Therefore, they are
chemically inert.
xxii. What do you know about oxyflourides of xenon?
Oxytetraflourides are colorless volatile liquid. It can be kept in nickel vessel. it reacts with
water to give XeO3. Xenon oxytetraflourides are prepared rapidly by the rapid reaction of
XeF6 with silica.
2XeF6 + SiO2 2XeF4 + SiF4
xxiii. Write down the names and formulas of ores of halogens.
Ores of fluorine are Fluorspar (CaF), Cryolite (Na3AlF6) and Apatite [(CaF2.3Ca3(PO4)2].
Ores of chlorine are Halite (NaCl), Carnallite (KCl.MgCl2.6H2O) (salt beds, brine wells, sea
water).
Ores of bromine are Brine wells, sea water, NaBr, KBr and MgBr2.
Ores of iodine are NaIO3, NaIO4 deposits in Chile brine walls.
xxiv. Give some chemical properties of Perchloric acid.
It reacts with organic compounds violently.
Dissolving power of HClO4 is enhanced due to increase in oxidizing strength.
Cold and dilute Perchloric acid is a very weak oxidizing agent.
It is the strongest of all acids in aqueous medium.
Q 3. Give the short answers of the any EIGHT questions.
16
xiii. Give the applications of noble gases.
Application of noble gases is
He is used in weather balloons, in welding and in traffic signal lights.
Ne and He arc is used in making glass lasers.
Xe is used in bactericidal lamps.
Radon being radioactive is used in radiotherapy for cancer and for earth quack prediction.](https://image.slidesharecdn.com/fsc-200803131854/85/F-sc-2-Chemistry-Ch-05-Solved-Test-Malik-Xufyan-4-320.jpg)




![F.sc.2.Chemistry.Ch.05. Solved Test (Malik Xufyan)
Cell # 0313-7355727
Exercise
Q 7. (a) How the halogen acids are ionized in water.
Halogen acids are ionized in water to give H+
ions.
HX + H2O H3O+
+ OH-
The order of acid strength is given below.
HF > HCl > HBr > HI
Q 13. Give the short answers of the following.
(i) What is “Iodized salt”?
When some amount of iodide ions added in common salt (NaCl), this is called iodized salt.
Usually, for this purpose NaI or KI is mixed with NaCl. Insufficient amount of iodine causes
the enlargement of thyroid glands.
(ii) What are Ferons and Teflon?
Flourochlorocarbons are called ferons. These are CCl2F2 (diflourodichloromethane) & CClF3
(tifluorochloromethane). These gases are used as refrigerant and aerosol poll ants. Teflon is a
polymer of ( [ CF2-CF2 ]n ). It is an important plastic.
(iii) Arrange the following ions in order of increasing size.
F-
, Cl-
, I-
, Br-
I-
> Br-
> Cl-
> F-
Ionic radii increase downward from top to bottom in a group. F-
Has smallest size while I-
has greater size.
(iv) Why iodine has metallic lustre?
Metallic luster in iodine is due to the excitation of electrons of iodine at room temperature.
Due to the bigger size of iodine the electrons of iodine at room temperature take energy and
go to higher energy states. When excited electrons come back, they emit some radiations of
particular wavelength. Therefore, they appear as luster grayish black solid.
(v) Which halogen sublimes to violets vapours?
Iodine is grayish black solid and emits vapours of violet color.
(vi) Which halogen is used as an antiseptic?
Iodine is used as antiseptic in the form of iodine tincture and iodex.
(vii) Which halogen is used in water treatment to kill bacteria?
Chlorine is disinfectant and used to kill pathogenic bacteria. It is used in drinking water and
swimming pools as disinfectant.
(viii) Name the gas, which is used for earth quack prediction.
Radon is used for earth quack prediction.
(ix) Name the gas, which is used in bacterial lamps.
Xenon is used in bacterial lamps.](https://image.slidesharecdn.com/fsc-200803131854/85/F-sc-2-Chemistry-Ch-05-Solved-Test-Malik-Xufyan-9-320.jpg)