Embed presentation
Download to read offline
































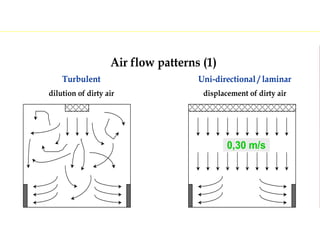

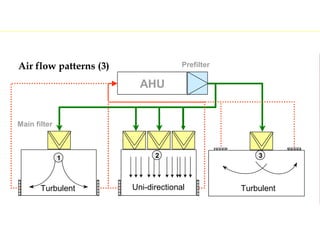

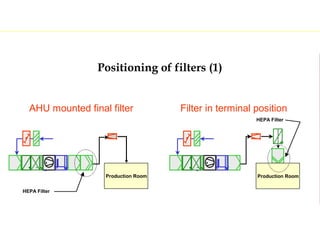
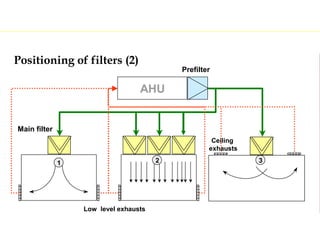
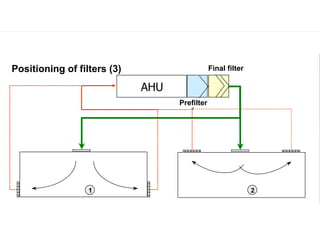

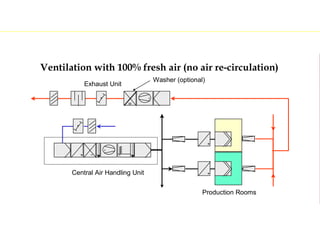
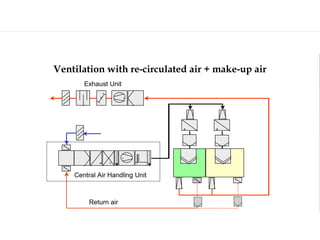
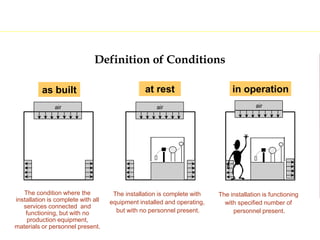

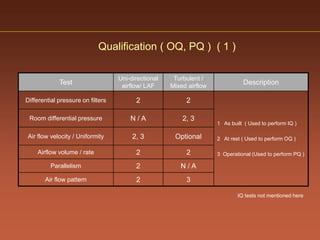
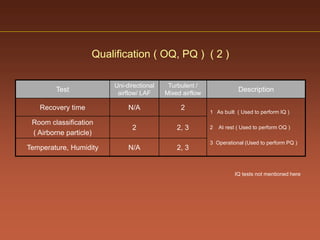
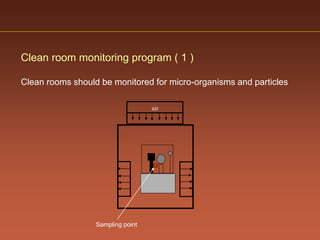
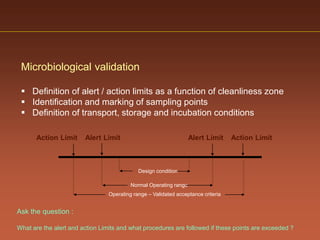


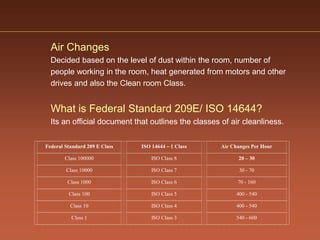


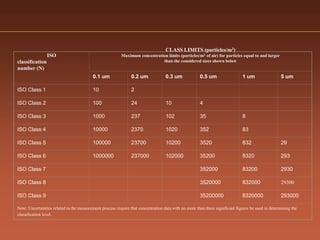
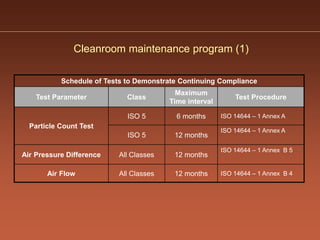
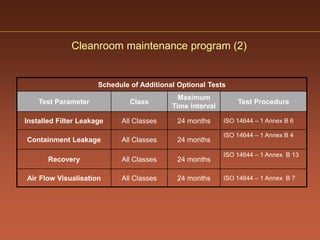




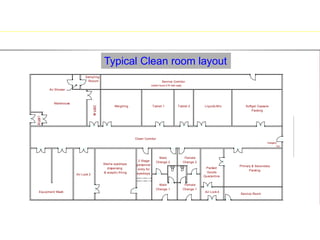


The document discusses current good manufacturing practices (cGMP) for active pharmaceutical ingredients (APIs). It lists major international codes of cGMP such as those from the WHO, EU, US FDA, ISO, and ICH. It also provides references and websites for cGMP standards. The document then discusses topics including validation, validation master plans, qualification types, calibration, facilities, equipment, air handling units, and containment practices.






























































