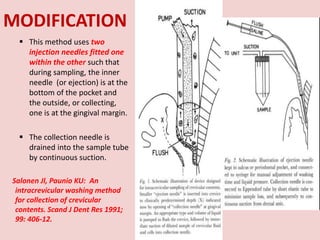
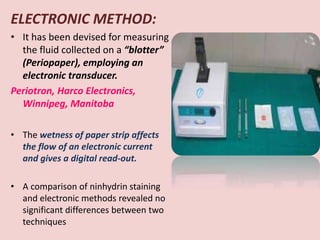
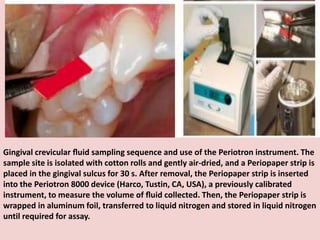
The document discusses the gingival sulcus and gingival crevicular fluid (GCF), detailing their anatomy, evaluation techniques, and the historical context of studies related to GCF. It highlights methods of fluid collection and analysis, emphasizing the importance of understanding GCF's role in oral health and disease. Various methodologies for sampling GCF, their advantages, disadvantages, and techniques for measurement, including electronic methods, are also elaborated upon.













































![• Neutrophilic enzymes in GCF:
PRIMARY / AZUROPHILIC
GRANULES
Elastase, cathepsin G, urokinase,
myeloperoxidase, lysozyme and
mannosidase, as well as
hydrolases active at acidic pH,
including cathepsin B, cathepsin
D and b-glucuronidase.
SECONDARY GRANULES
contain lactoferrin,
neutrophil collagenase
[matrix metalloproteinase-
8 (MMP-8)], and lysozyme](https://image.slidesharecdn.com/lgdbnzd0teeljxrm4kvu-signature-df6e6d3b4081953f62e2c31b36443d13284e4eb62d42d6e5cdcdb22b0e27810f-poli-170427193236/85/Gingival-crevicular-fluid-49-320.jpg)





















