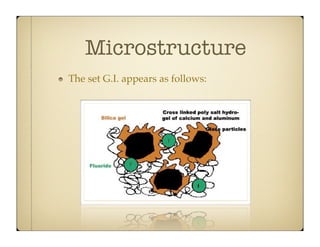
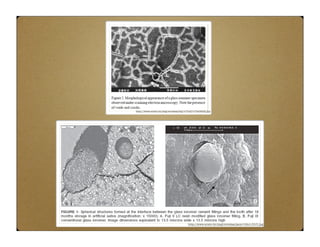
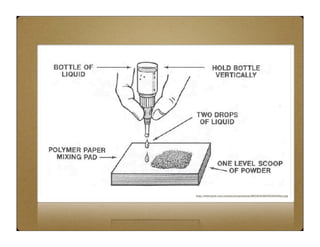
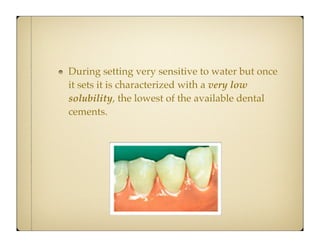
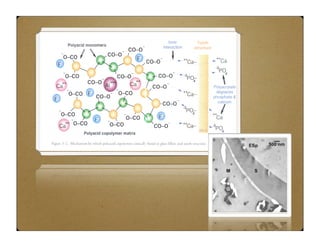
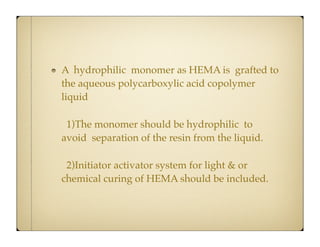
Glass ionomer cement (GIC) was developed to combine properties of silicate and polycarboxylate cements. It sets via an acid-base reaction between fluoroaluminosilicate glass powder and polyacrylic acid liquid. The setting reaction forms a matrix of hydrated calcium and aluminum polysalts surrounding unreacted glass particles. GIC has advantages like aesthetics, fluoride release, and chemical bonding to tooth structure. However, its early formulations had limitations like opacity, discoloration over time, and moisture sensitivity during setting. Modifications to GIC include resin-modified, cermet, compomer, and giomer to improve properties while maintaining benefits like fluoride release.


![Silicate Cements
It was introduced in~1870 as direct aesthetic
restoration [historical type]
Supplied form : Powder & Liquid
Composition:
Powder: Fluoro- calcium- aluminum- silicate
glass (ion leach-able glass )
Liquid: Aqueous solution of phosphoric acid](https://image.slidesharecdn.com/gic-140212221914-phpapp02/85/GIC-3-320.jpg)
![Setting Reaction
It is an acid base reaction
Base + Acid
Salt + Water
It is a surface reaction
cored structure
Advantages :
Aesthetic [glass content]
Anticariogenic [fluoride content]](https://image.slidesharecdn.com/gic-140212221914-phpapp02/85/GIC-4-320.jpg)
![Disadvantages
[Early clinical failure].
Highly irritant to the pulp due to.
- Its high acidity.
- Arsenic impurity content.
Highly soluble in oral fluids.](https://image.slidesharecdn.com/gic-140212221914-phpapp02/85/GIC-5-320.jpg)