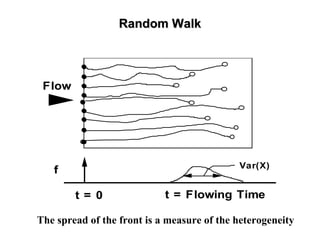
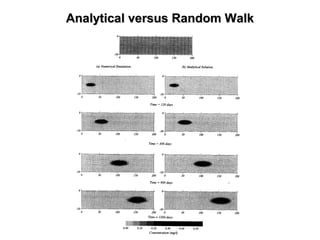
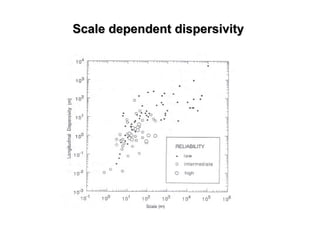
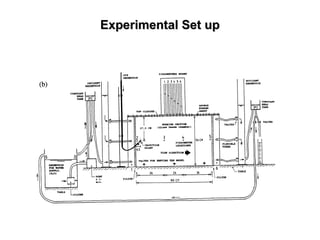
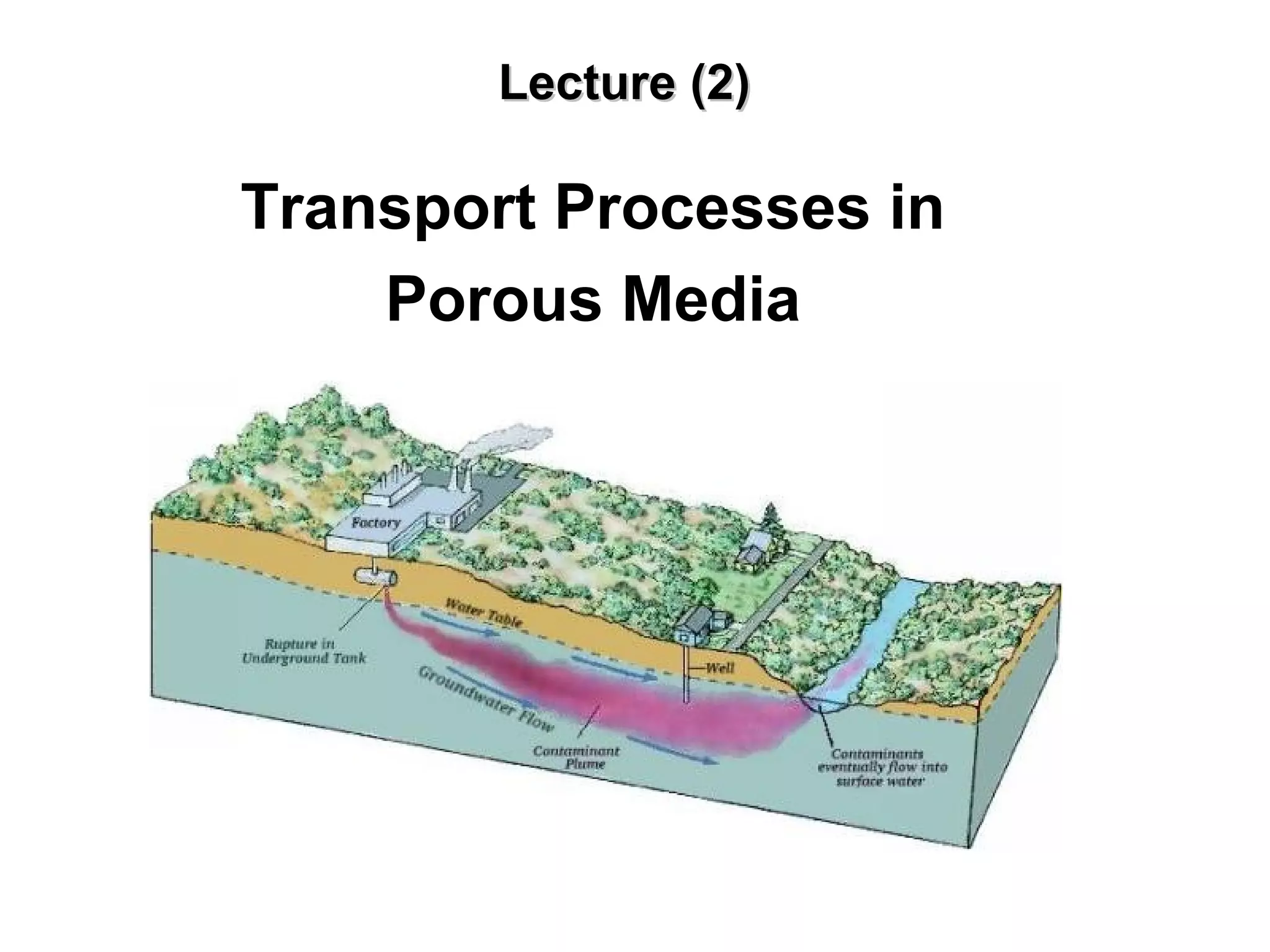
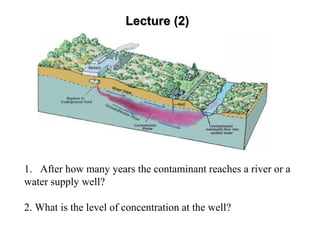
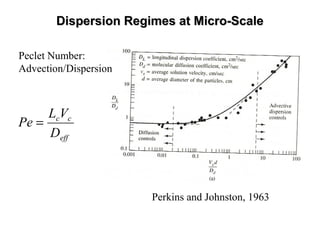
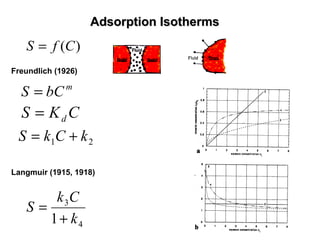
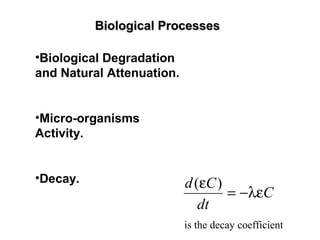
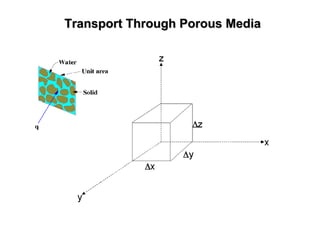
The lecture discusses transport processes in porous media, covering contaminant movement, concentration levels, and the derivation of the advective dispersion equation. It highlights physical, chemical, biological processes and methods of solution including analytical and numerical approaches. Key topics include advection, diffusion, dispersion, adsorption, and decay, with a focus on their effects on pollutant transport.



![Mechanical DispersionMechanical Dispersion
dis
J = - Cε ∇.D
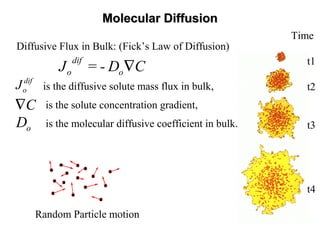
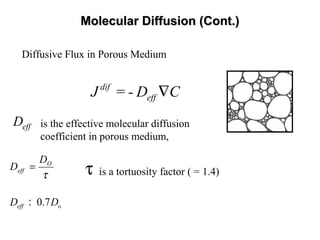
Depressive Flux in Porous Media (Fick’s Law):
is the depressive solute mass flux,
is the solute concentration gradient,
is the dispersion tensor,
is the effective porosity
dis
J
ε
C∇
D
xx xy xz
yx yy yz
zx zy zz
D D D
D D D
D D D
=
D
[after Kinzelbach, 1986]
Causes of Mechanical Dispersion](https://image.slidesharecdn.com/geohydrologyii2-170217165654/85/Geohydrology-ii-2-9-320.jpg)
![Hydrodynamic DispersionHydrodynamic Dispersion
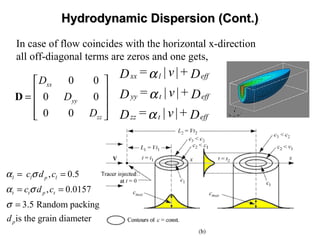
( ) ( ) i j
ij efft ij l t
v v
= |v|+ + -D D
|v|
α δ α α
_hydo dis
J = - Cε ∇.D
Hydrodynamic Depressive Flux in Porous Media (Fick’s Law):
The components of the dispersion tensor in isotropic soil
is given by [Bear, 1972],
is Kronecker delta, =1 for i=j and =0 for i≠ j,ijδ
are velocity components in two perpendicular directions,i jv v
is the magnitude of the resultant velocity,v 2 2 2
i j kv v v v= + +
is the longitudinal pore-(micro-) scale dispersivity, andlα
tα is the transverse pore-(micro-) scale dispersivity
ijδ ijδ](https://image.slidesharecdn.com/geohydrologyii2-170217165654/85/Geohydrology-ii-2-10-320.jpg)

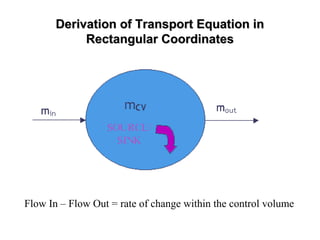
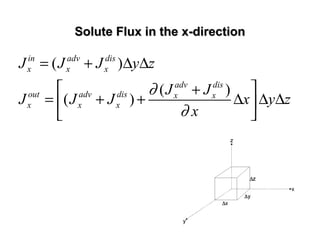
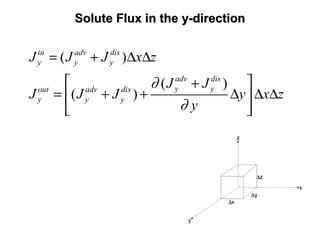
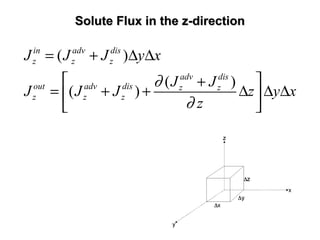
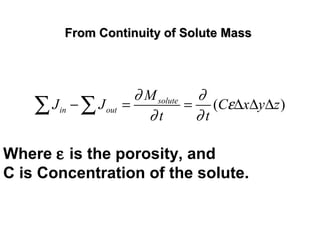
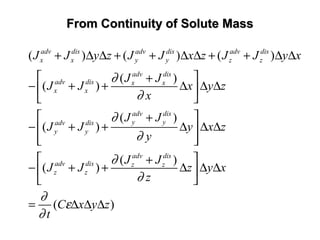
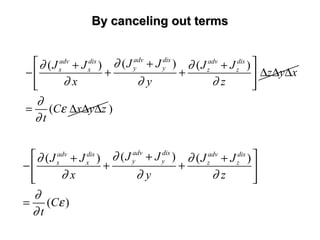
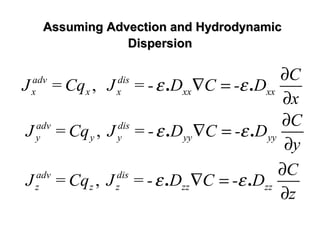
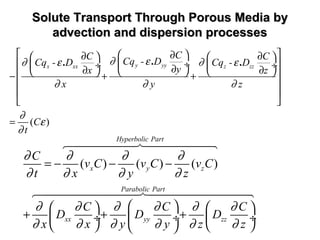
![General Form of The Transport EquationGeneral Form of The Transport Equation
[ ]
}
}
/
( ')
Dispersion Diffusion
Advection Source SinkChemical reaction
Decay
ij i
i j i
C C S C C W
v C + Q CD
t x x x
λ
ε ε
−
−
∂ ∂ ∂ ∂ −
= − − +
∂ ∂ ∂ ∂
6447448 64748 64748
where
C is the concentration field at time t,
Dij
is the hydrodynamic dispersion tensor,
Q is the volumetric flow rate per unit volume of the source or sink,
S is solute concentration of species in the source or sink fluid,
i, j are counters,
C’ is the concentration of the dissolved solutes in a source or sink,
W is a general term for source or sink and
vi
is the component of the Eulerian interstitial velocity in xi
direction
defined as follows,
ij
i
j
K
= -v
xε
∂Φ
∂
where
Kij
is the hydraulic conductivity tensor, and ε is the porosity of the medium.](https://image.slidesharecdn.com/geohydrologyii2-170217165654/85/Geohydrology-ii-2-26-320.jpg)
![Schematic Description of ProcessesSchematic Description of Processes
Figure 7. Schematic Description of the Effects of Advection, Dispersion, Adsorption, and Degradation on Pollution
Transport [after Kinzelbach, 1986].
Advection+Dispersion
Advection
Advection+Dispersion+Adsorption
Advection+Dispersion+Adsorption+Degradation](https://image.slidesharecdn.com/geohydrologyii2-170217165654/85/Geohydrology-ii-2-27-320.jpg)

![Pulse versus Continuous InjectionPulse versus Continuous Injection
Concentration Distribution in case of Pulse and Continuous Injections in a 2D Field
[after Kinzelbach, 1986].
tV4
)Y-(y
+
tV4
)tV-X-(x
-
tV4tV4
H)/(M
=t)y,C(x,
xt
2
o
xl
2
xo
xtxl
o
αααπαπ
ε
exp ( )
d
tV4
Y-y
+
tV4
tV-X-x
-
tV4
HM=ty,x,C
t
xt
2
o
xl
2
xo
tlx
o
∫
−−
−
−
•
0
)(
)(
)(
)((
exp
1)(/
)( τ
τατα
τ
τααπ
ε](https://image.slidesharecdn.com/geohydrologyii2-170217165654/85/Geohydrology-ii-2-29-320.jpg)