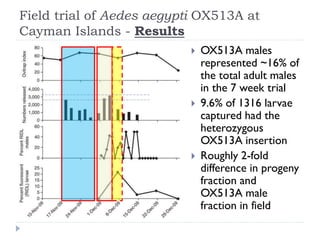
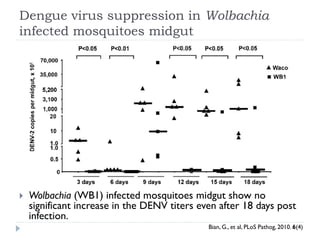
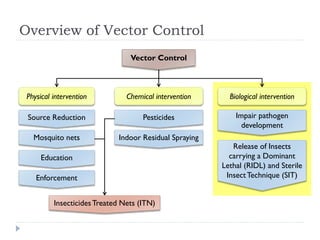
This document provides an overview of genetically modified mosquitoes for vector control. It discusses the mosquito lifecycle and transmission of vector-borne diseases. Methods for vector control include the use of Wolbachia-infected mosquitoes, which have shown promise in suppressing dengue virus in laboratory and field trials by impairing pathogen development. The document also describes techniques using sterile insects like the sterile insect technique (SIT) and release of insects carrying a dominant lethal gene (RIDL). Field trials on the Cayman Islands demonstrated that Wolbachia-infected mosquitoes can successfully introduce and spread the infection within a native mosquito population. However, more studies are still needed before GM mosquitoes can be effectively used for vector control.





















![Science behind RIDL
Oxitec uses a piggyBac transposon construct in their GM
mosquitoes which is as shown in the picture below
piggyBac is a stable transposase system which is widely adopted in many
cancer and insect studies
tTAV component is conjoined with a female specific sterility gene
[fs(1)K10] – to achieve single sex population
fs(1)K10 is required in the dorsal-ventral patterning of the embryo
and over-expression will result in progeny having double dorsal
regions, and not surviving past the fourth –instar larval stage
LA513 construct Phuc Hk et al, BMC Biol. 2007 Mar 20; 5:11](https://image.slidesharecdn.com/eae01e27-e580-4c84-ac6c-967713da73e7-150606124320-lva1-app6892/85/Genetically-modified-mosquitoes-25-320.jpg)