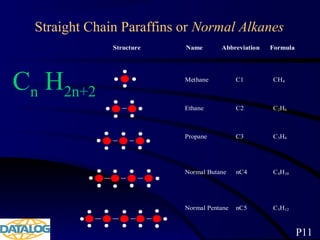
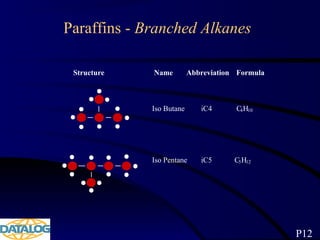
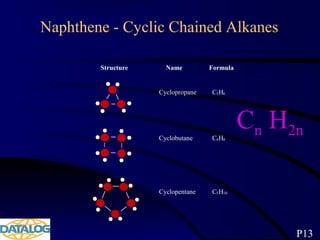
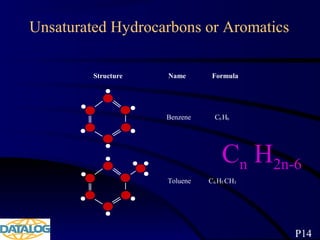
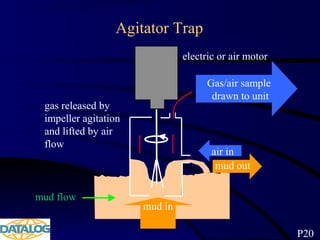
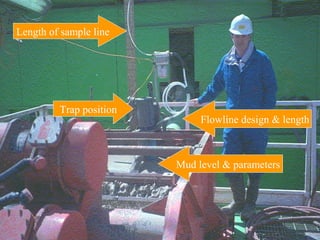
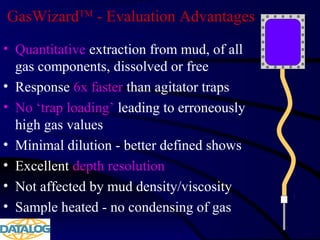
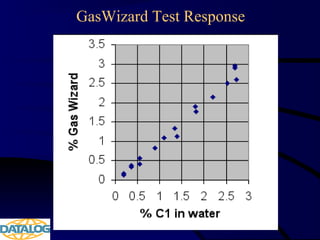
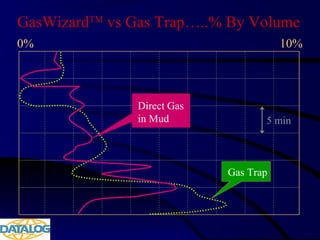
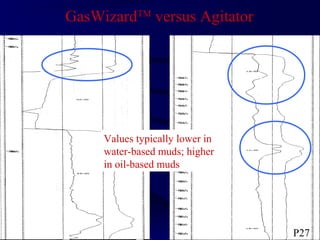
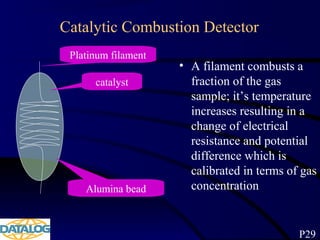
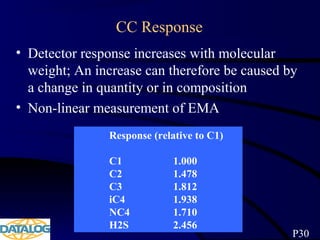
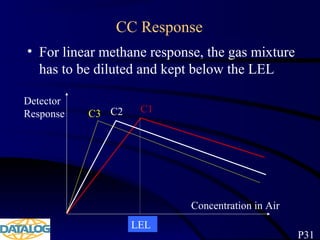
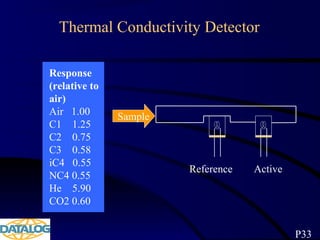
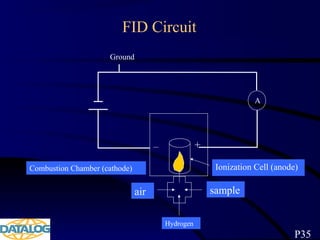
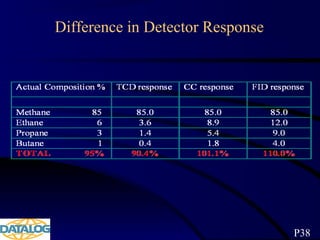
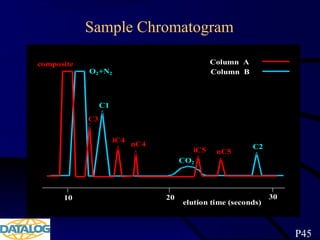
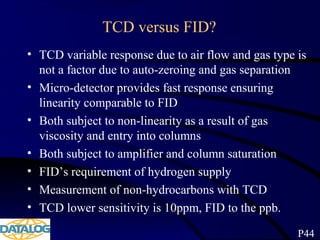
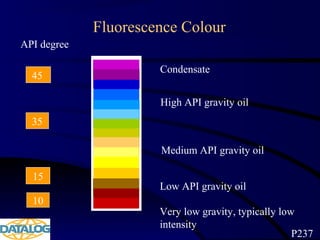
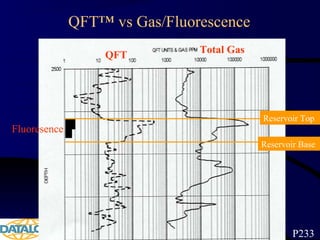
The document provides an overview of a course on hydrocarbon evaluation and interpretation from wellsite gas measurements. It discusses various gas measurement techniques including agitator traps, direct gas measurement tools, and chromatographic analysis. It describes the operation of common gas detectors like catalytic combustion, thermal conductivity, and flame ionization. It also covers limitations and applications of total gas detection versus chromatography. The document aims to help users understand gas responses and properly interpret real-time wellsite data for evaluation of zones, fluid typing, and reservoir properties.