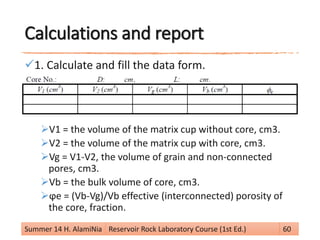
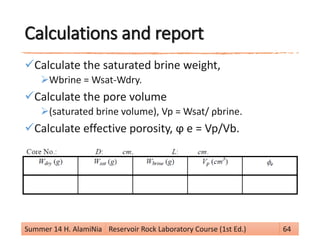
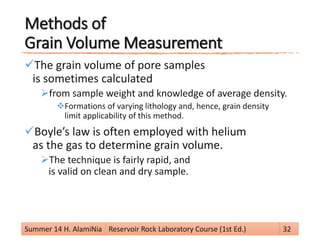
This document provides an overview of reservoir rock porosity determination methods. It defines total, effective, and dead porosity. It describes techniques for measuring bulk volume, including through dimensions, fluid displacement, and mercury injection. It discusses methods for determining grain volume, such as crushing samples and using a pycnometer or volumeter. The document emphasizes that knowing two of the three values (bulk volume, grain volume, or pore volume) is required to calculate porosity.



































![Gas Expansion Method
Many porosimeters are designed to use the
principle of Boyle’s law of gas expansion to
determine the grain volume.
The idea is to allow the remaining volume of a chamber
in which a core is placed (V1 – Vg) at pressure P1 to
expand by an additional volume V2 and read the final
pressure P2.
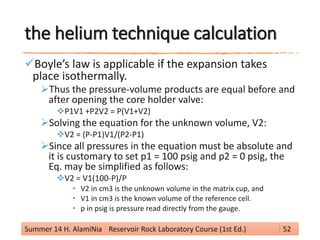
From Boyle’s Law (at constant temperature).
(V1 – Vg) P1 = (V1 – Vg + V2) P2
knowing V1, V2, P1 and P2 allows the calculation of grain
volume Vg.
Vg = V1 – [(P2 / (P1 – P2)] V2
Summer 14 H. AlamiNia Reservoir Rock Laboratory Course (1st Ed.) 38](https://image.slidesharecdn.com/yxw4btacqhuyvnqim8kk-signature-214a99b049e91446322d8a6671d9629efb4e7db18e62cfb94b2fb26c56651ea2-poli-140817171617-phpapp01/85/Q923-rrl-l04-38-320.jpg)










![Gas Expansion Method
The mercury pump (with a vacuum) gauge is used.
After the bulk volume is determined and mercury
fills the chamber but does not penetrate the sample,
the air in the pores is allowed to expand by withdrawing
the mercury from the chamber.
If the volume of mercury withdrawn is V which is read
on the pump scale then from Boyle’s Law:
Vp P1 = (Vp + V) P2 So: Vp = V[(P2 / (P1 – P2)]
• P2 is the final pressure read on the vacuum gauge and
• P1 is initial pressure (atmospheric)
It is clear that if P2 = ½ P1 then Vp = V
• So the pore volume would be equal to the volume of mercury
withdrawn from the chamber to reduce the pressure in the
chamber to half its original (atmospheric) value.
Summer 14 H. AlamiNia Reservoir Rock Laboratory Course (1st Ed.) 49](https://image.slidesharecdn.com/yxw4btacqhuyvnqim8kk-signature-214a99b049e91446322d8a6671d9629efb4e7db18e62cfb94b2fb26c56651ea2-poli-140817171617-phpapp01/85/Q923-rrl-l04-49-320.jpg)