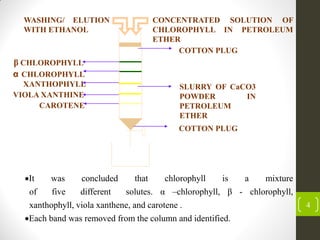
Michael Tswett discovered chromatography in 1901 when he separated chlorophyll, a green pigment found in plants, into five distinct bands or solutes (α-chlorophyll, β-chlorophyll, xanthophyll, viola xanthene, and carotene) using a glass column packed with calcium carbonate (CaCO3) powder and eluting with ethanol. The separation occurred because the solutes migrated down the column at different velocities due to having different degrees of adsorption to the CaCO3 stationary phase, from least adsorption (highest velocity) for carotene to greatest adsorption (lowest velocity) for β-chlorophyll. This demonstrated that chromatography can separate mixtures and defined the basic components and process of column