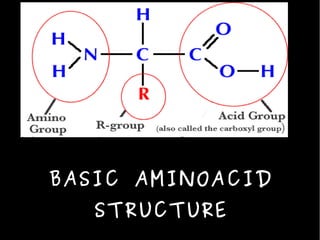
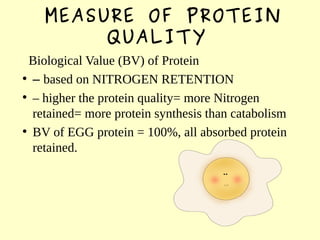
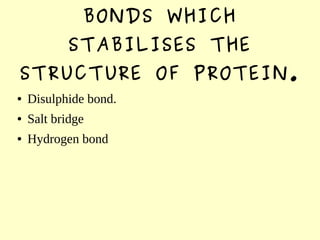
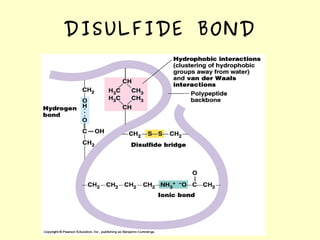
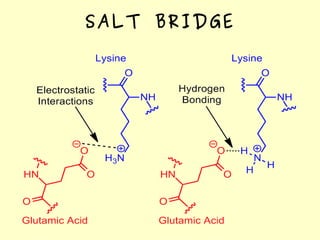
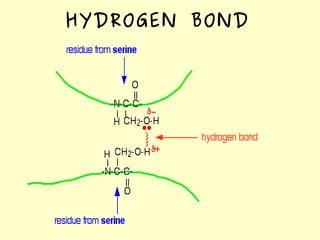
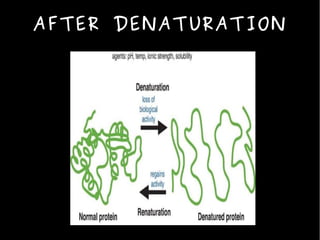
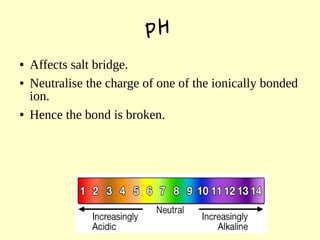
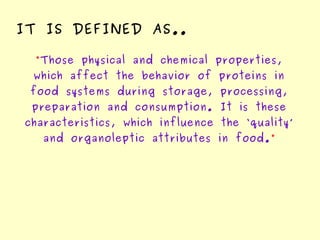
The document discusses the sources, structures, and functional properties of proteins, highlighting the differences between plant and animal proteins in terms of digestibility and amino acid completeness. It explains protein denaturation, its causes, and the effects on solubility and functionality in food systems, such as gelation, emulsification, and foaming. Additionally, it touches on the factors affecting protein quality and characteristics critical to food processing and preparation.