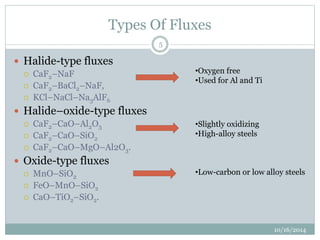
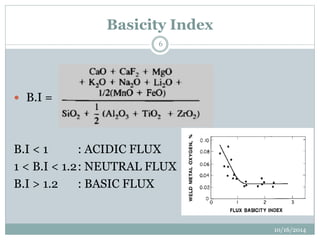
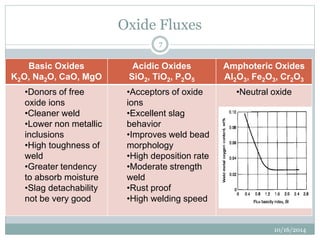
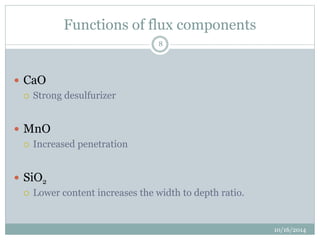
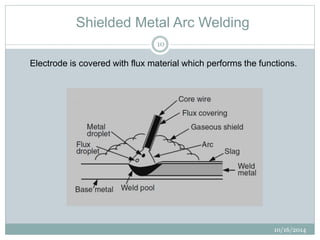
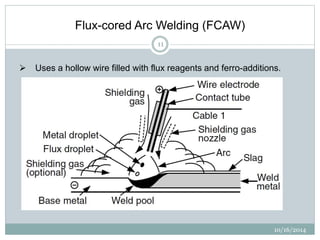
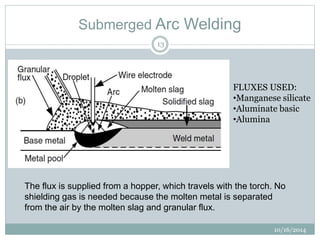
The document discusses the various roles and types of fluxes in welding, highlighting their importance in preventing oxide formation, improving arc stability, and enhancing weld quality. It details different flux compositions, such as halide, oxide, and their functions, as well as the desired properties of slag formed during welding. Additionally, it describes specific welding techniques that utilize these fluxes, such as shielded metal arc welding and flux-cored arc welding.