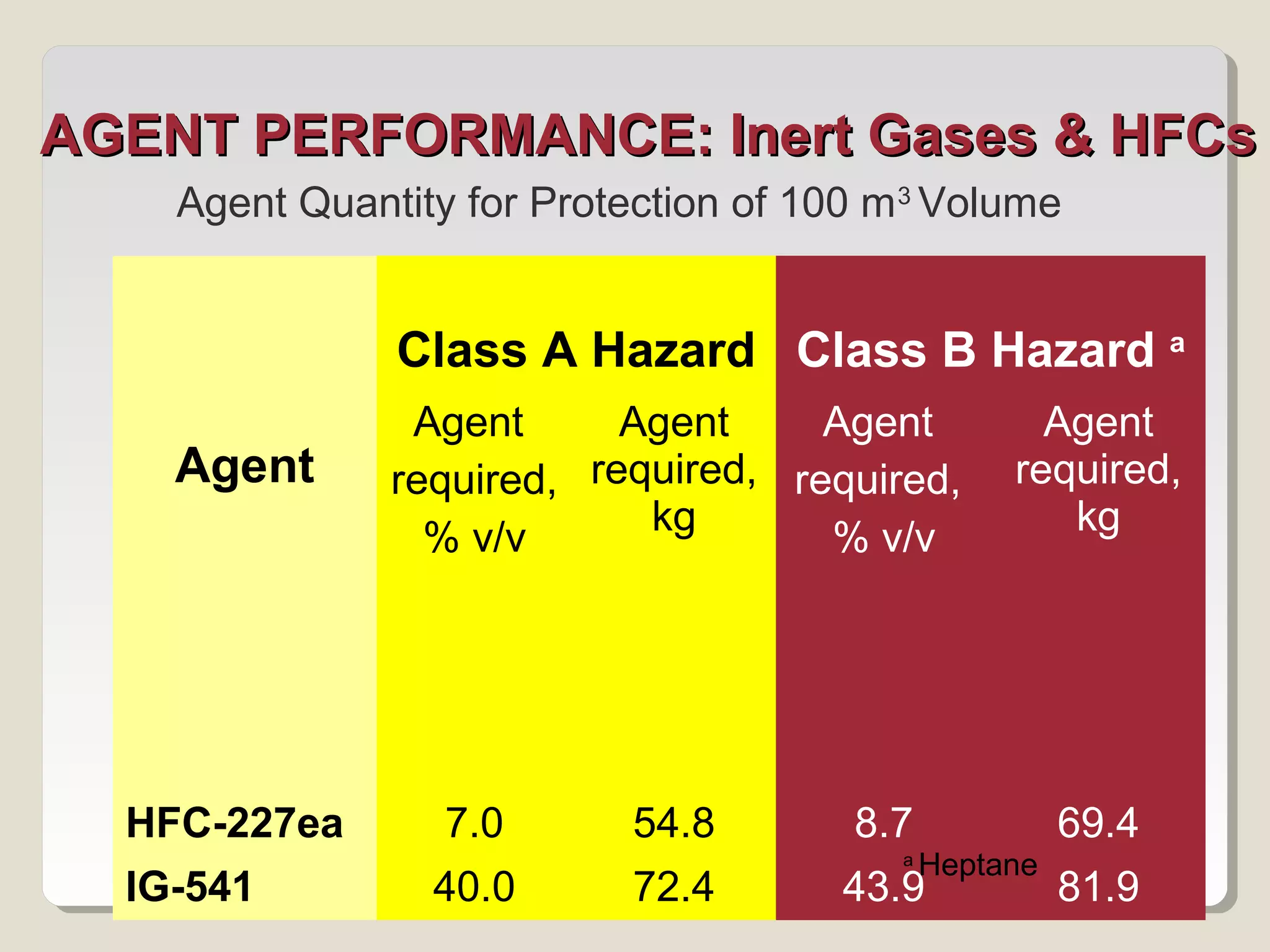
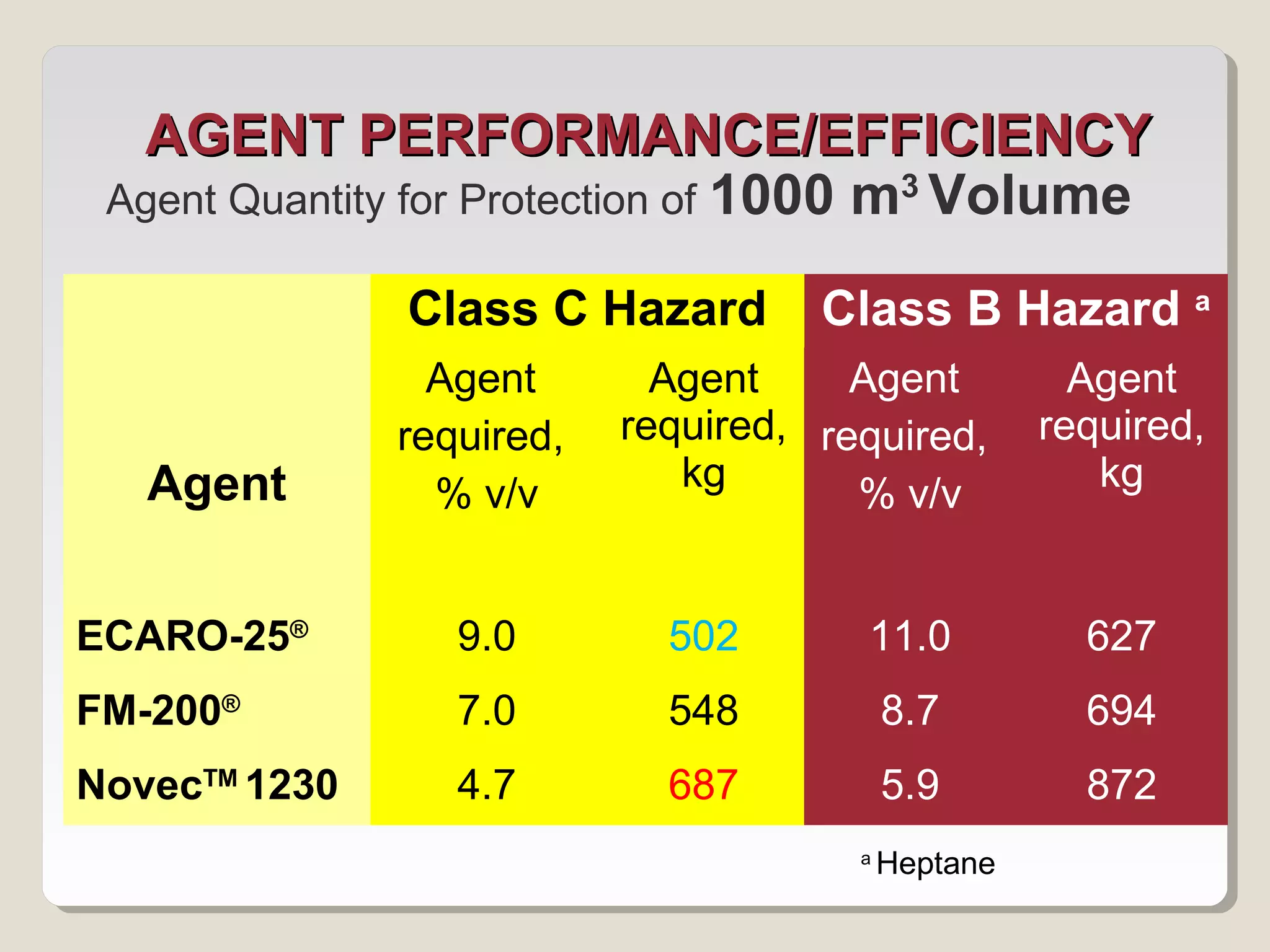
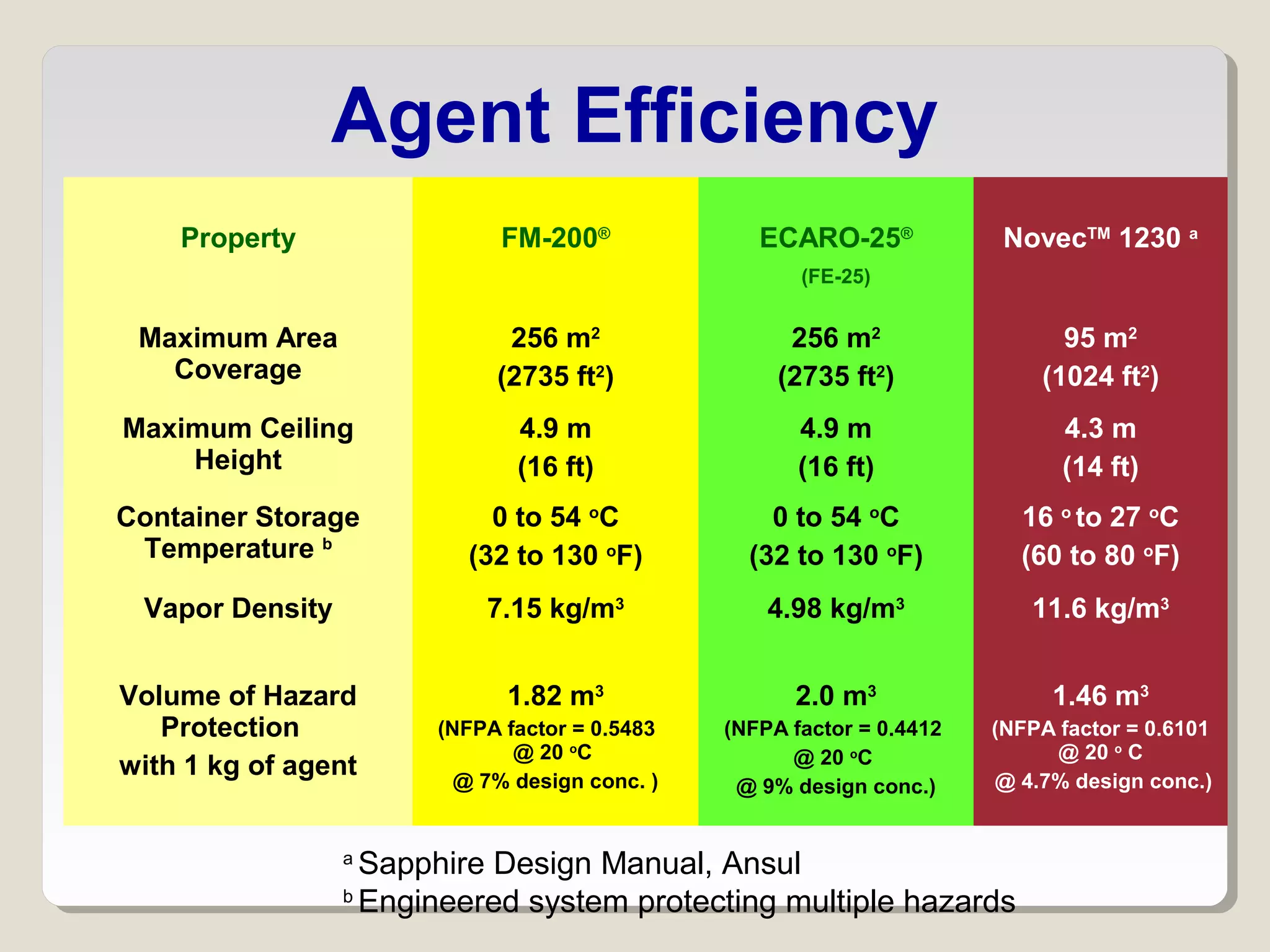
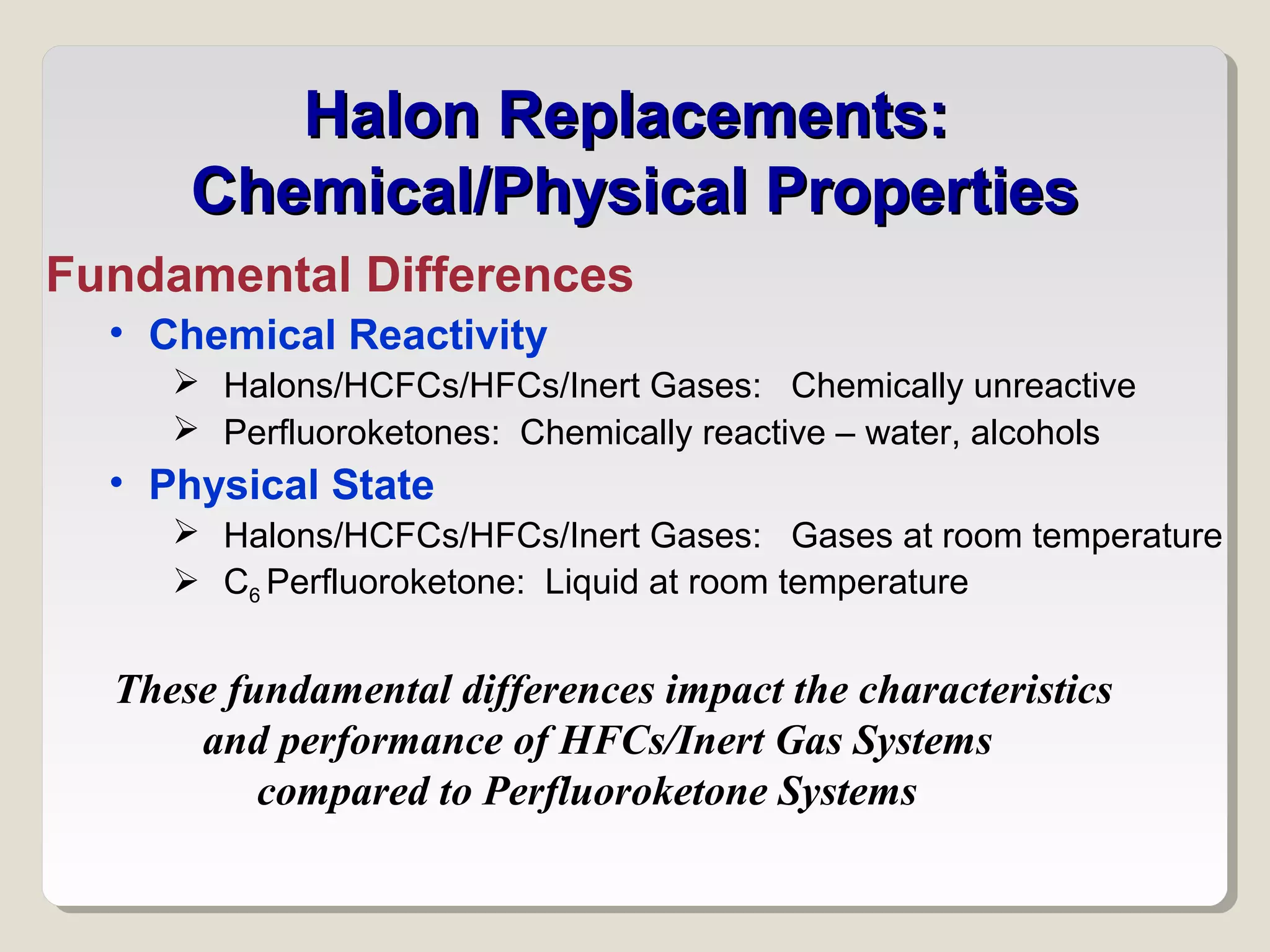
The document discusses halon total flooding fire suppression agents, highlighting the advantages of halon 1301 and its replacements, such as hydrofluorocarbons (HFCs), hydrochlorofluorocarbons (HFCs), inert gases, and perfluorinated ketones. It outlines the configurations, performance metrics, and chemical properties of these agents, emphasizing the importance of low chemical reactivity for optimal effectiveness in fire suppression. Additionally, it addresses environmental impact, toxicity, and implications of chemical interactions of the various agents on performance and safety.













![Perfluoropropionic AcidPerfluoropropionic Acid
CF3CF2COOH
Strong Acid/Extremely Corrosive
• Is a perfluorocarboxylic acid – among strongest acids known
• Attacks steel, forming iron salt [DuPont, 2004]
Toxic [MSDS]
• Causes eye and skin burns
• May cause severe & permanent damage to digestive tract
• Causes gastrointestinal burns
• Causes chemical burns to respiratory tract
• PFCAs known tumor promoters [Env. Sci. Tech 2005, 39, 5517]
• PFCAs are known peroxisome proliferators [Ibid.]
Liver damage](https://image.slidesharecdn.com/systemcomparison-fmecaro-25vsnovec1230-180117163128/75/Fire-System-comparison-fm-ecaro-25-vs-novec-1230-20-2048.jpg)





![Chemical Reactivity
Requirements FM-200®
/
ECARO-25®
NovecTM
1230
Low Chemical
Reactivity
√ Characterized by high
chemical reactivity
No reaction with
alcohols, amines √ Reacts with alcohols, amines
No reaction with
solvents √
Incompatible with polar or
hydrocarbon solvents
“Additional work is needed to assess the
reactivity of C6K [NovecTM
1230]…” a
Source: 3M: 24thIEEE SEMI-THERM Symposium, 2008, page 173.](https://image.slidesharecdn.com/systemcomparison-fmecaro-25vsnovec1230-180117163128/75/Fire-System-comparison-fm-ecaro-25-vs-novec-1230-26-2048.jpg)