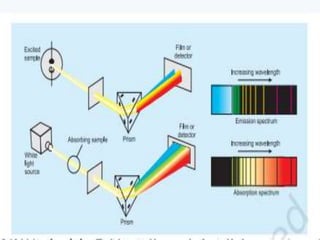
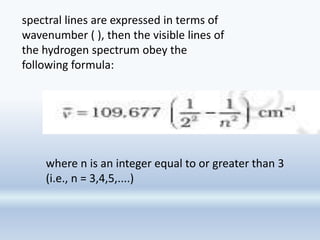
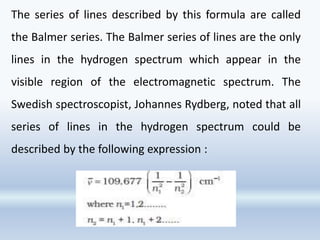
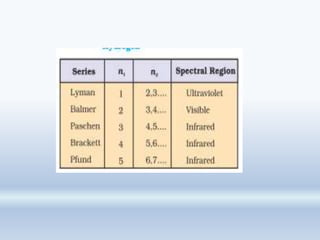
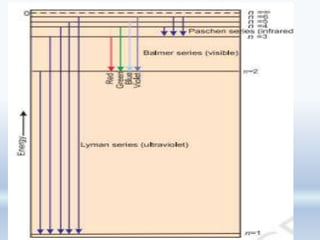
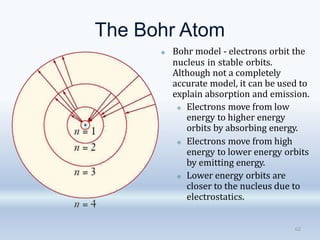
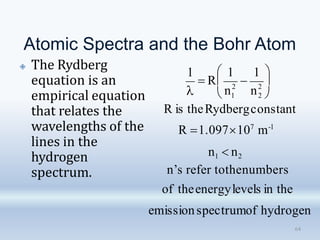
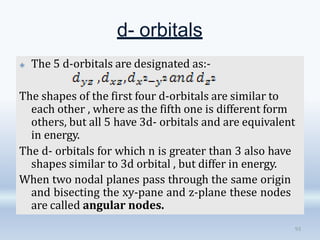
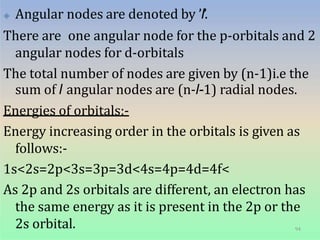
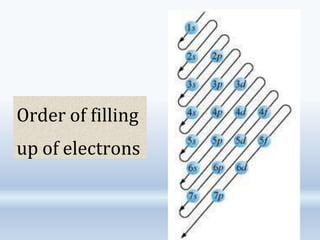
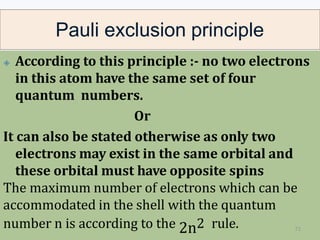
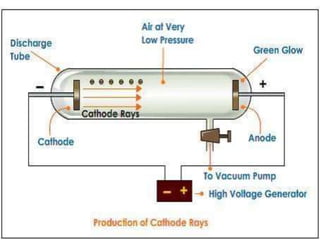
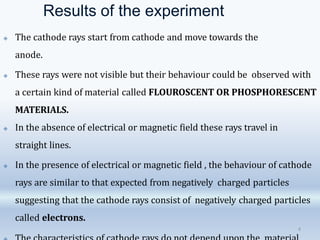
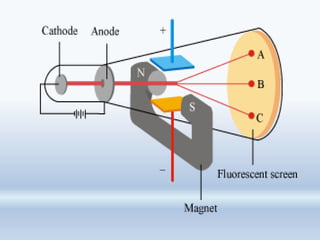
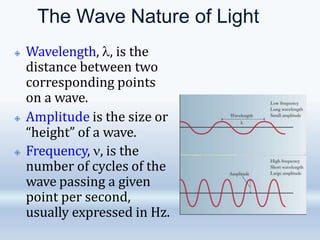
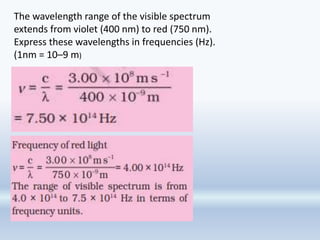
1. The document discusses the discovery of the electron through cathode ray experiments and the determination of the charge to mass ratio of electrons.
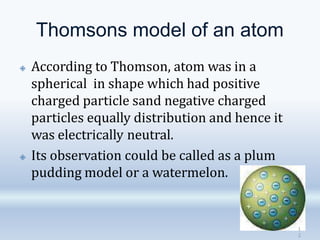
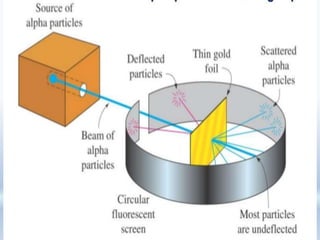
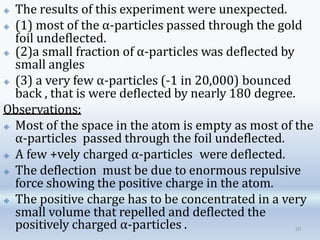
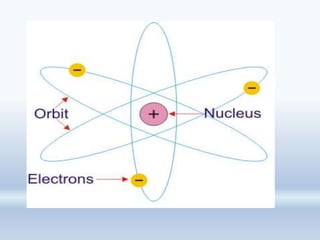
2. Rutherford's alpha particle scattering experiments showed that the atom has a small, dense nucleus containing positive charge and mass, surrounded by electrons. This led to the development of the Rutherford model of the atom.
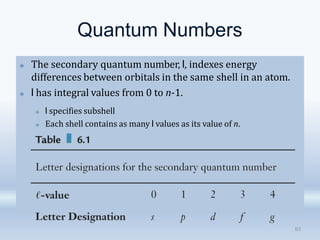
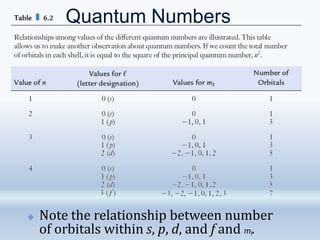
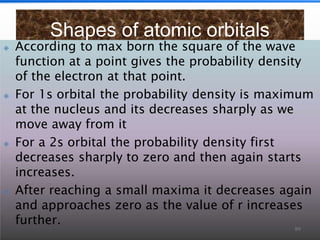
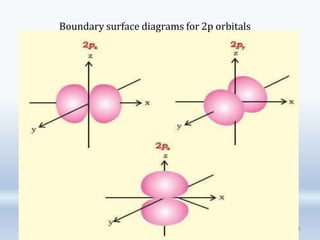
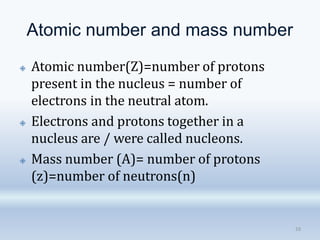
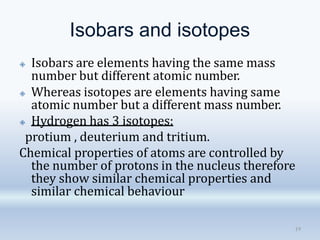
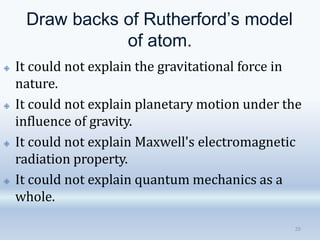
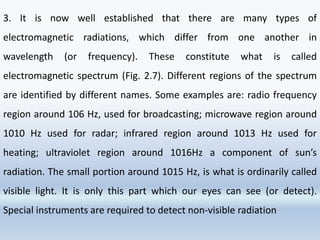
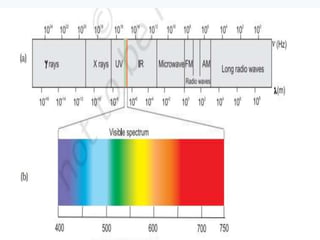
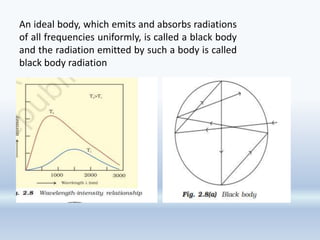
3. The document also discusses the discovery of protons and neutrons, atomic number and mass number, isotopes, drawbacks of the Rutherford model, wave-particle duality of light, and Planck's quantum theory.














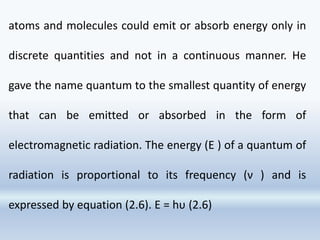


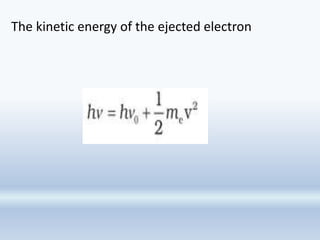
![It has been observed that though the number of
electrons ejected does depend upon the brightness of
light, the kinetic energy of the ejected electrons does
not. For example, red light [ν = (4.3 to 4.6) × 1014 Hz] of
any brightness (intensity) may shine on a piece of
potassium metal for hours but no photoelectrons are
ejected. But, as soon as even a very weak yellow light (ν
= 5.1–5.2 × 1014 Hz) shines on the potassium metal, the
photoelectric effect is observed. The threshold
frequency (ν 0 ) for potassium metal is 5.0×1014 Hz](https://image.slidesharecdn.com/chapter2-structureofatom2023-230917194529-6a28b4ba/85/Chapter2-Structure-of-Atom-2023-pptx-42-320.jpg)