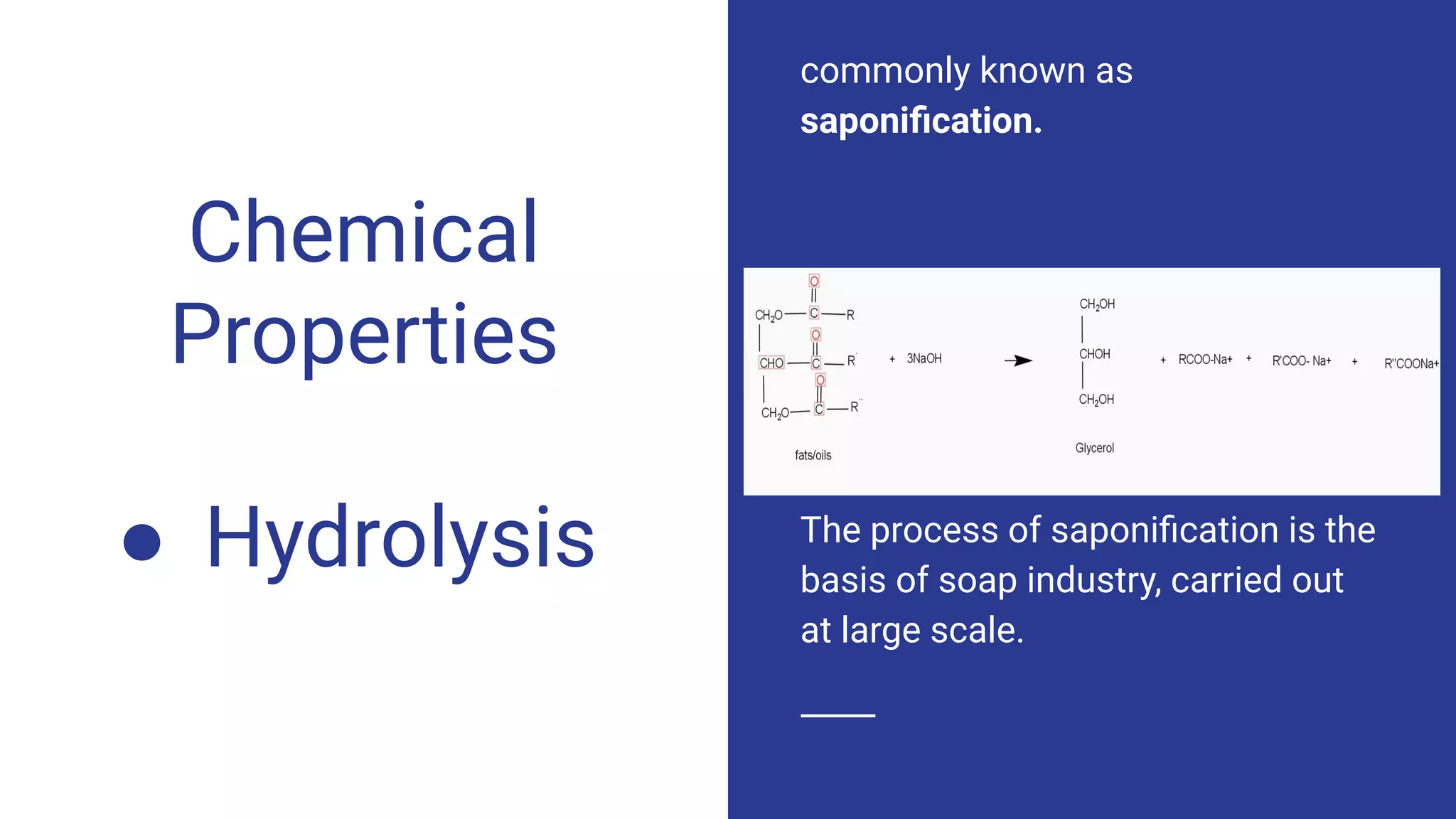
The document discusses fats, oils, and detergents, highlighting their chemical properties, types, and applications. It defines natural and synthetic fats and oils, their hydrolysis and hydrogenation processes, and introduces key chemical constants like acid, saponification, and iodine values. Additionally, it distinguishes between soaps and synthetic detergents, elaborating on their compositions and functionalities.