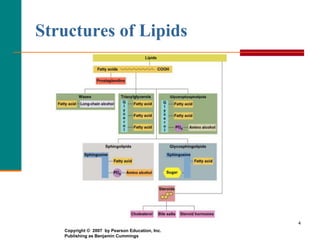
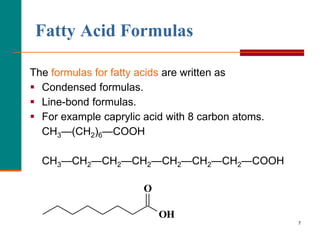
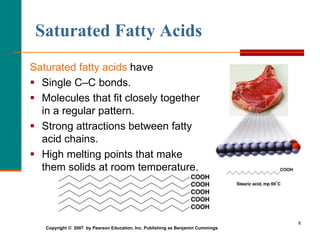
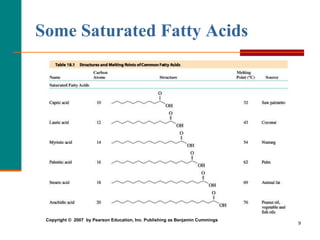
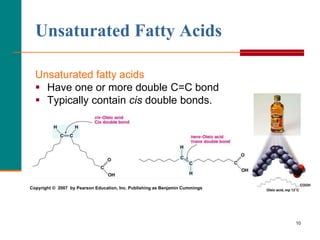
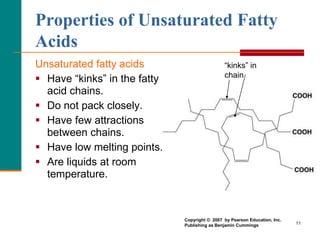
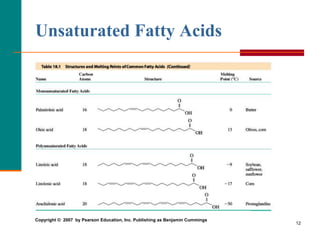
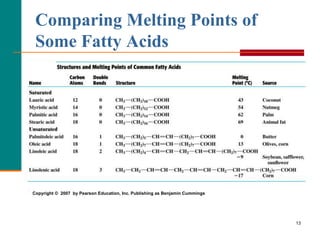
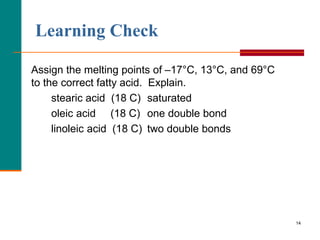
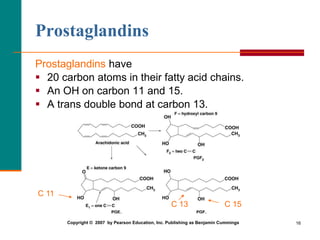
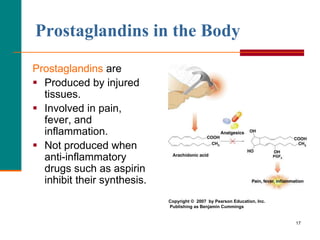
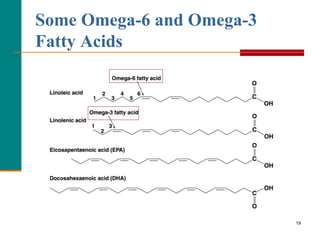
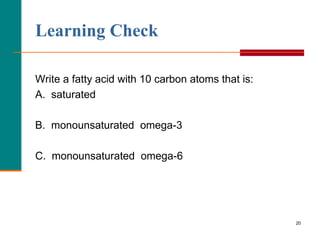
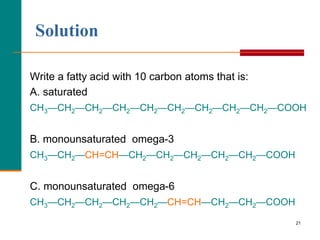
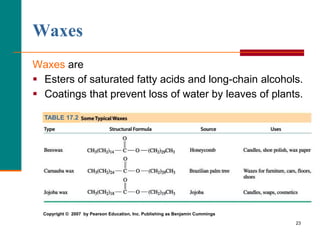
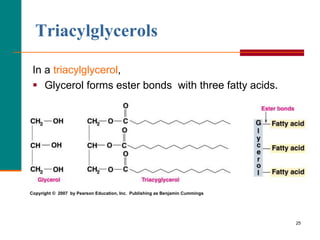
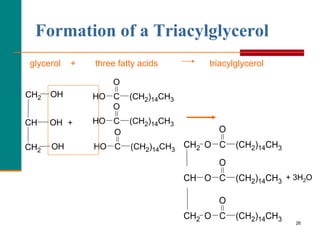
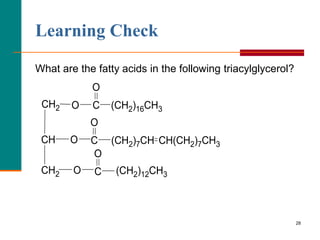
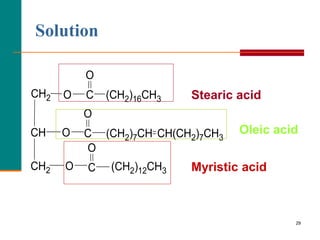
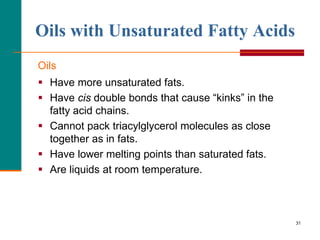
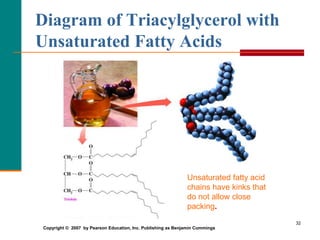
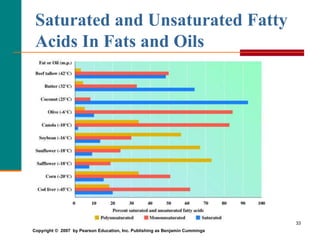
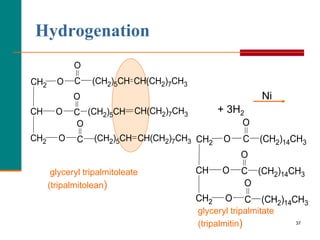
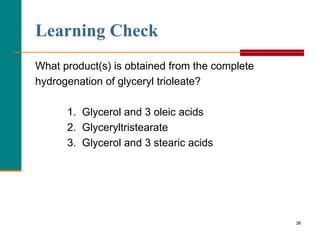
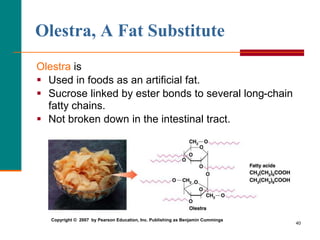
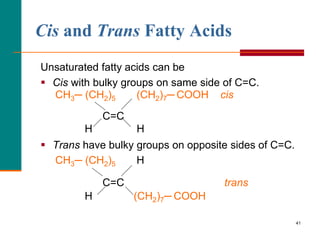
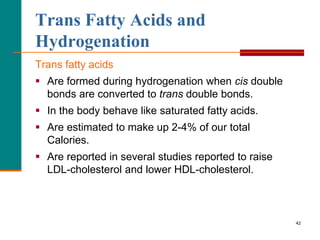
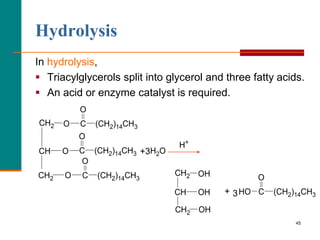
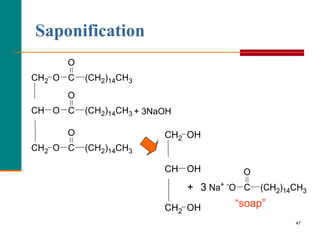
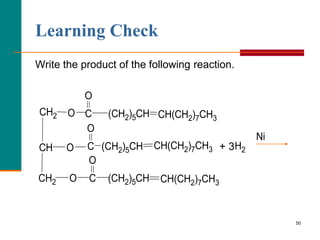
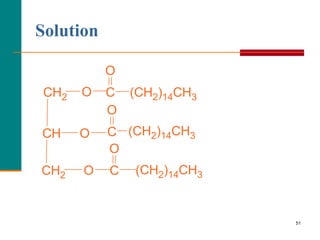
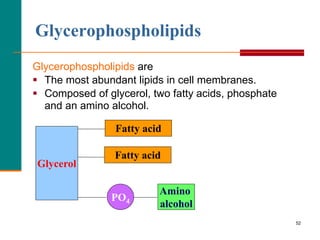
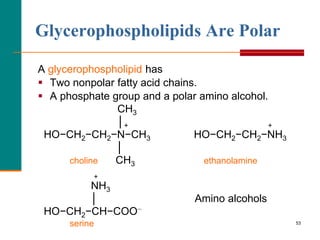
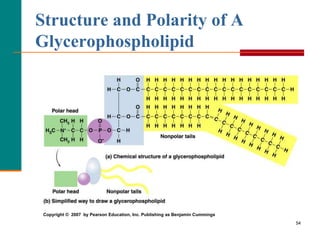
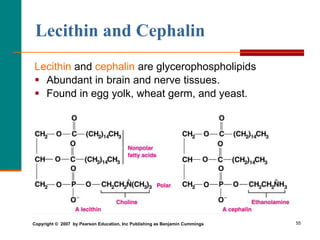
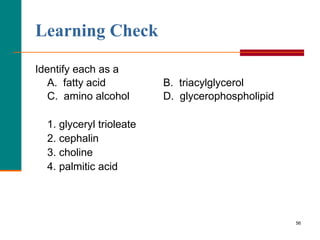
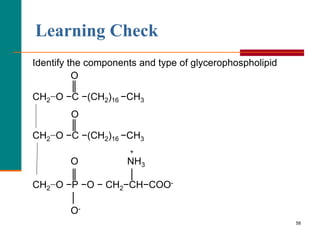
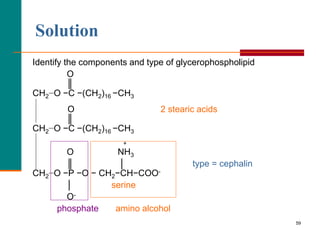
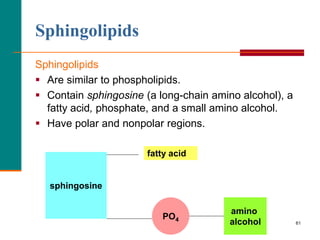
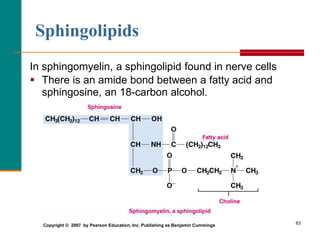
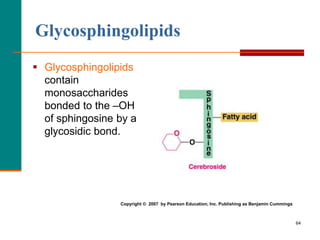
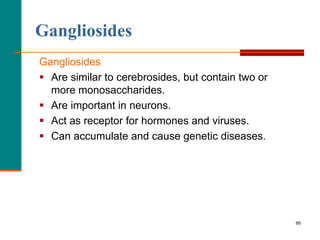
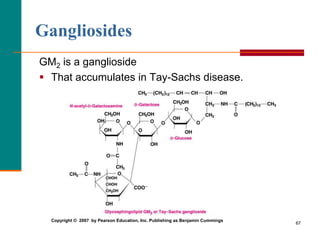
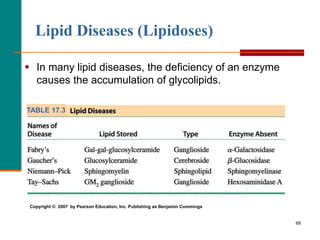
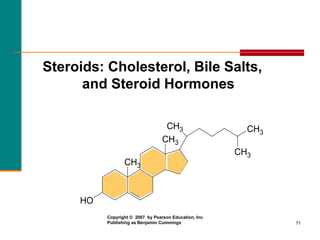
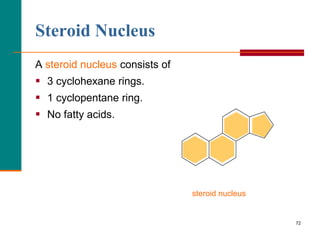
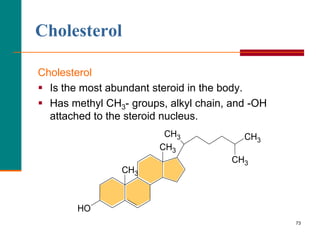
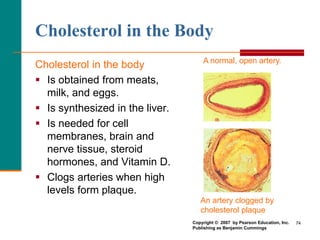
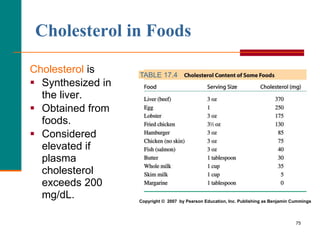
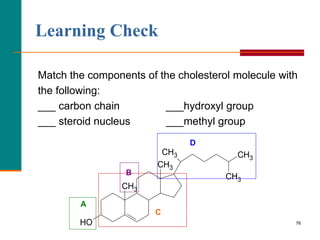
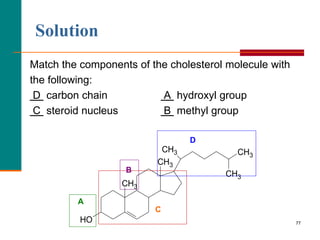
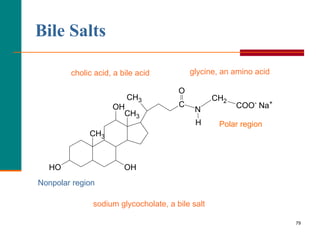
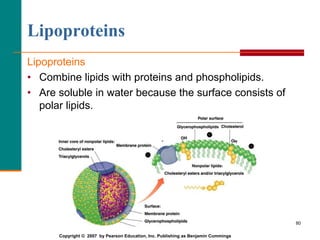
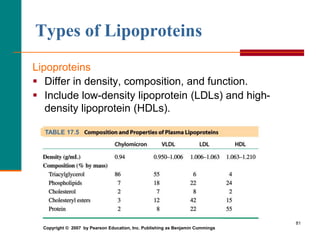
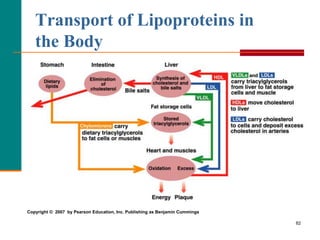
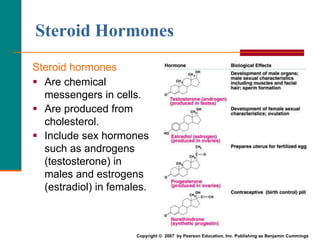
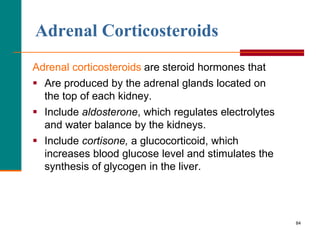
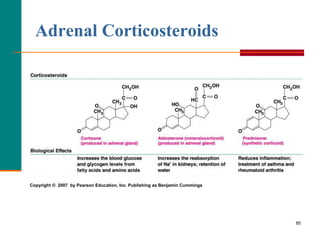
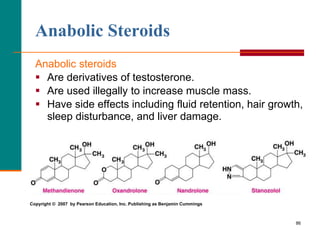
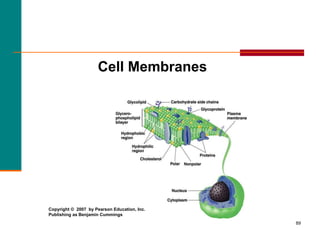
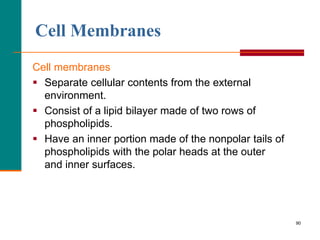
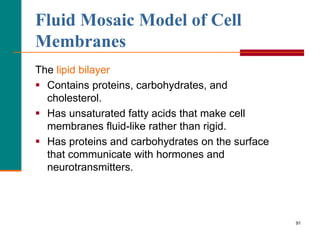
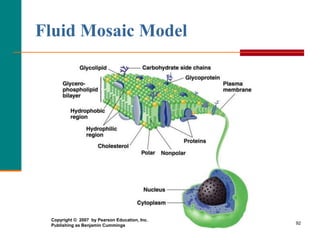
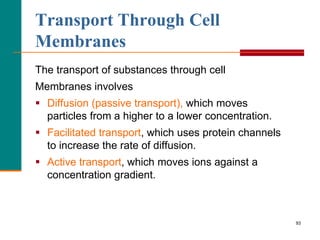
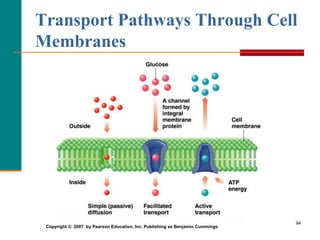
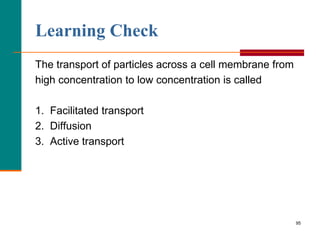
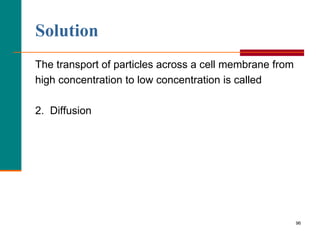
This document provides information about lipids and fatty acids. It defines lipids as biomolecules that contain fatty acids or a steroid nucleus and are soluble in organic solvents but not water. There are different types of lipids containing fatty acids, including waxes, fats and oils (triacylglycerols), glycerophospholipids, and prostaglandins. Fatty acids are long-chain carboxylic acids that can be saturated or unsaturated. Fats and oils are esters of glycerol and three fatty acids called triacylglycerols. Unsaturated fatty acids have kinks that prevent close packing, giving oils and unsaturated fats lower melting points than saturated fats. Hydrogen