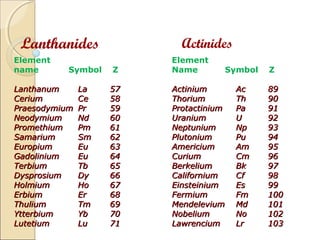
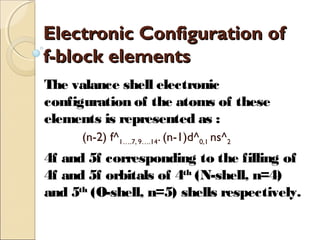
The document discusses f-block elements, which are elements where extra electrons fill the (n-2)f orbitals. They are classified into two blocks: lanthanides which have electrons filling the 4f orbitals, and actinides which have electrons filling the 5f orbitals. The lanthanides include elements from lanthanum to lutetium, which have electronic configurations of (n-2)f1-14(n-1)d0-1ns2. The actinides include elements from actinium to lawrencium. They are radioactive, show various oxidation states, and react with water and acids to produce hydrogen gas.