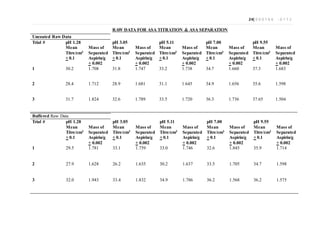
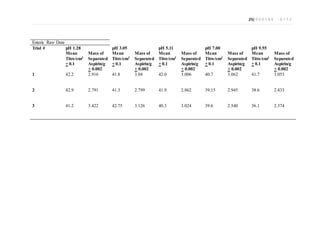
This document is an extended essay chemistry experiment investigating how different tablet coatings affect the dissolution of aspirin in various pH levels. The student prepared pH solutions replicating stomach and intestinal pH and dissolved uncoated, buffered, and enteric coated aspirin tablets in them. They then used back titration to calculate the percentage of aspirin dissolved. The results showed enteric coated tablets dissolved significantly less than uncoated and buffered tablets in stomach pH, demonstrating the coatings protected the aspirin. Uncoated and buffered tablets dissolved more uniformly across all pH levels.