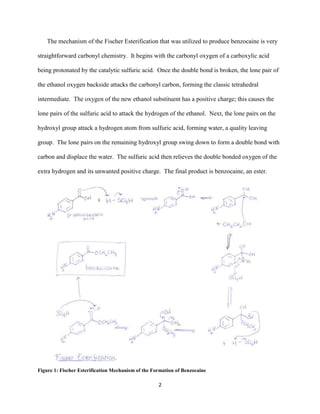
Esters are important compounds that are used in many synthetic reactions to produce products for medicinal, cosmetic, and fuel applications. This document details a laboratory experiment where benzocaine, a local anesthetic, was synthesized from p-aminobenzoic acid using Fischer esterification. The product was analyzed and characterized using melting point, NMR, IR, and GC-MS to confirm it was benzocaine. The percent yield of 85.8% indicated a successful synthesis.