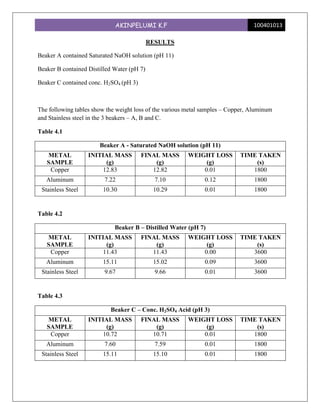
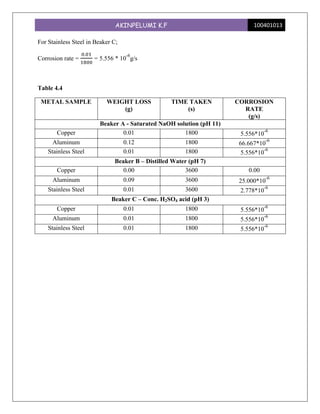
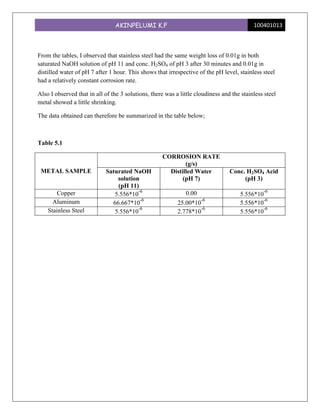
This experiment investigated the effect of pH levels on corrosion rates of copper, aluminum, and stainless steel. The metals were immersed in solutions with pH levels of 11, 7, and 3. Aluminum showed the highest corrosion rates, which increased with higher pH levels. Copper had no corrosion at pH 7 but equal rates at pH 11 and 3. Stainless steel had consistent low corrosion rates across pH levels. The experiment demonstrated that pH significantly impacts corrosion for different metals, with aluminum being most susceptible.


![AKINPELUMI K.F 100401013
INTRODUCTION
The aim of this experiment is to investigate the effect of pH level on corrosion rate. The
necessity of this experiment arises from the need to demonstrate how a potentially corrosive
situation may be recognized and avoided.
pH, originally defined by Danish biochemist Søren Peter Lauritz Sørensen in 1909, is a measure
of the concentration of hydrogen ions. The term pH was derived from the manner in which the
hydrogen ion concentration is calculated, it is the negative logarithm of the hydrogen ion (H+)
concentration:
pH = -log [H+] …………………………….eqn 3.1
Where log is a base-10 logarithm. According to the Compact Oxford English Dictionary, the "p"
stands for the German word for "power", potenz, so pH is an abbreviation for "power of
hydrogen".
A higher pH means there are fewer free hydrogen ions, and that a change of one pH unit reflects
a tenfold change in the concentrations of the hydrogen ion. For example, there are 10 times as
many hydrogen ions available at a pH of 7 than at a pH of 8. The pH scale ranges from 0 to 14.
A pH of 7 is considered to be neutral. Substances with pH of less than 7 are acidic and
substances with pH greater than 7 are considered to be basic.
Low pH acid waters clearly accelerate corrosion by providing a plentiful supply of hydrogen
ions. Although even absolutely pure water contains some free hydrogen ions, free carbon dioxide
in the water can multiply the hydrogen ion concentration many times. When carbon dioxide
dissolves in water, it reacts with the water to form carbonic acid, a so-called weak acid, but an
effective source of acidity. Even more acidity is sometimes encountered in acid mine waters, or
in those contaminated with industrial wastes.
Both acids and alkali's have the capability of being corrosive, although one would have a pH
range of 0 - (acid), while the other would range in the area of 14 (alkali). Sodium hydroxide, a
very strong and corrosive alkali would have the same damaging effect on human tissue as
sulfuric acid. If a 25% concentration of sulfuric acid and phosphoric acid were measured for pH,
both would range in the area of 0. However, if sulfuric acid were allowed to contact human
tissue, severe burns would result, while the average person would not detect even a burning
sensation from contact with the phosphoric acid. Why? The answer lies in the corrosive nature of
some acids over others.](https://image.slidesharecdn.com/koredeexp5labreport-130112130319-phpapp01/85/effect-of-pH-level-on-corrosion-rate-3-320.jpg)