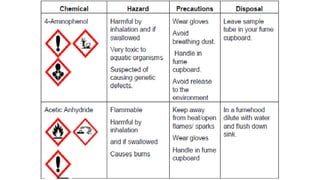
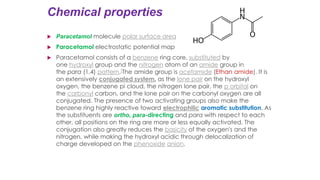
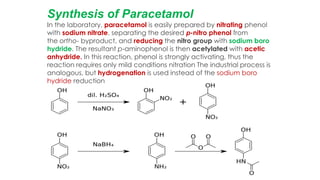
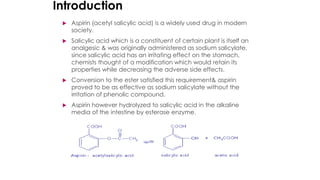
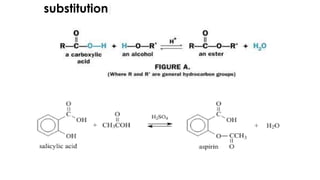
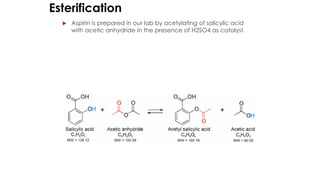
The document classifies pharmaceutical products into solids, semi-solids, liquids, and gases and describes common examples of each type such as tablets, creams, syrups, and inhalers. It then discusses the manufacturing process for solid oral dosage forms like tablets and capsules which involves steps like blending, granulation, compression, and coating. Key equipment used includes drying equipment, tablet presses, coating machines, and quality control instruments.