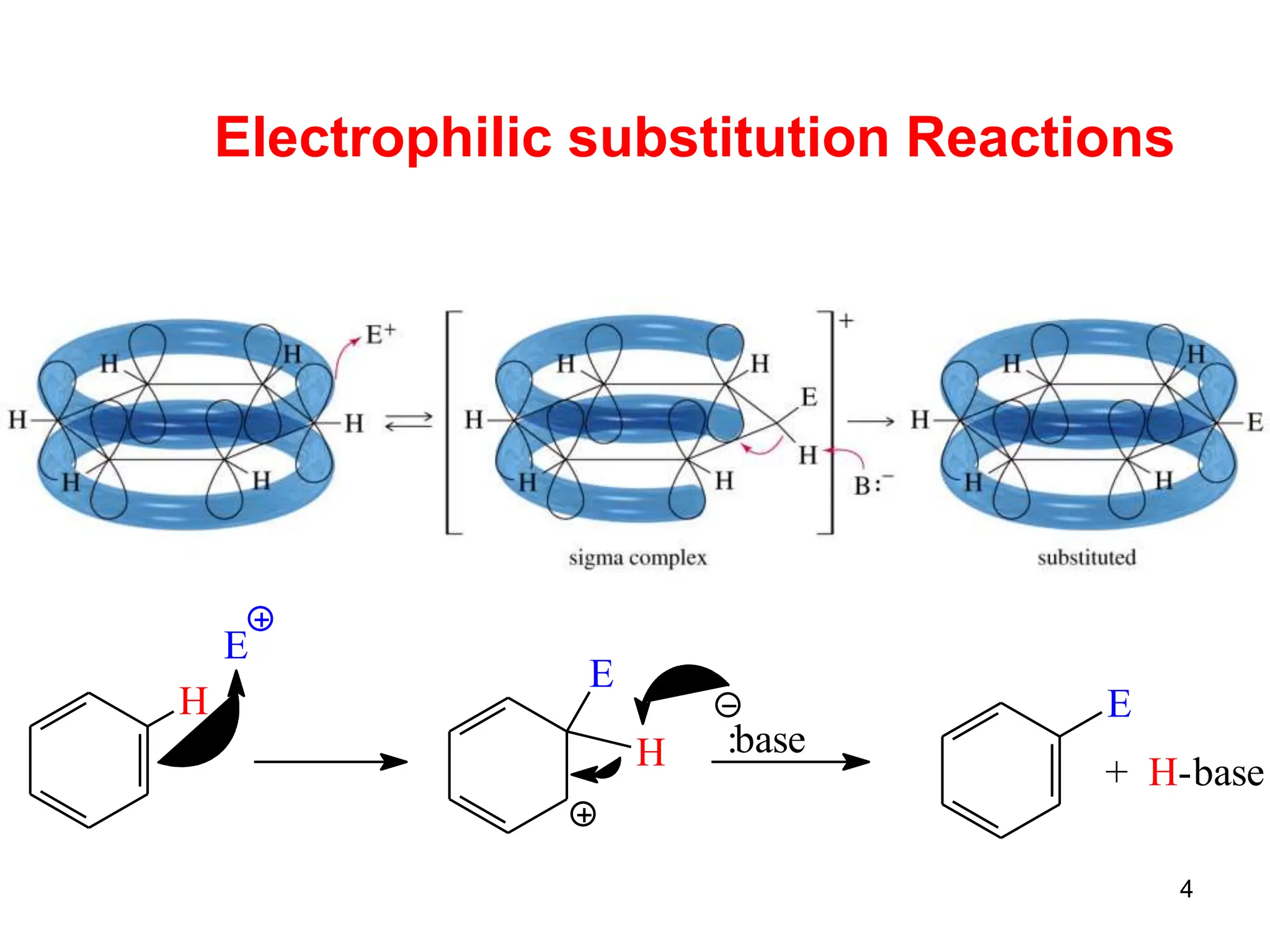
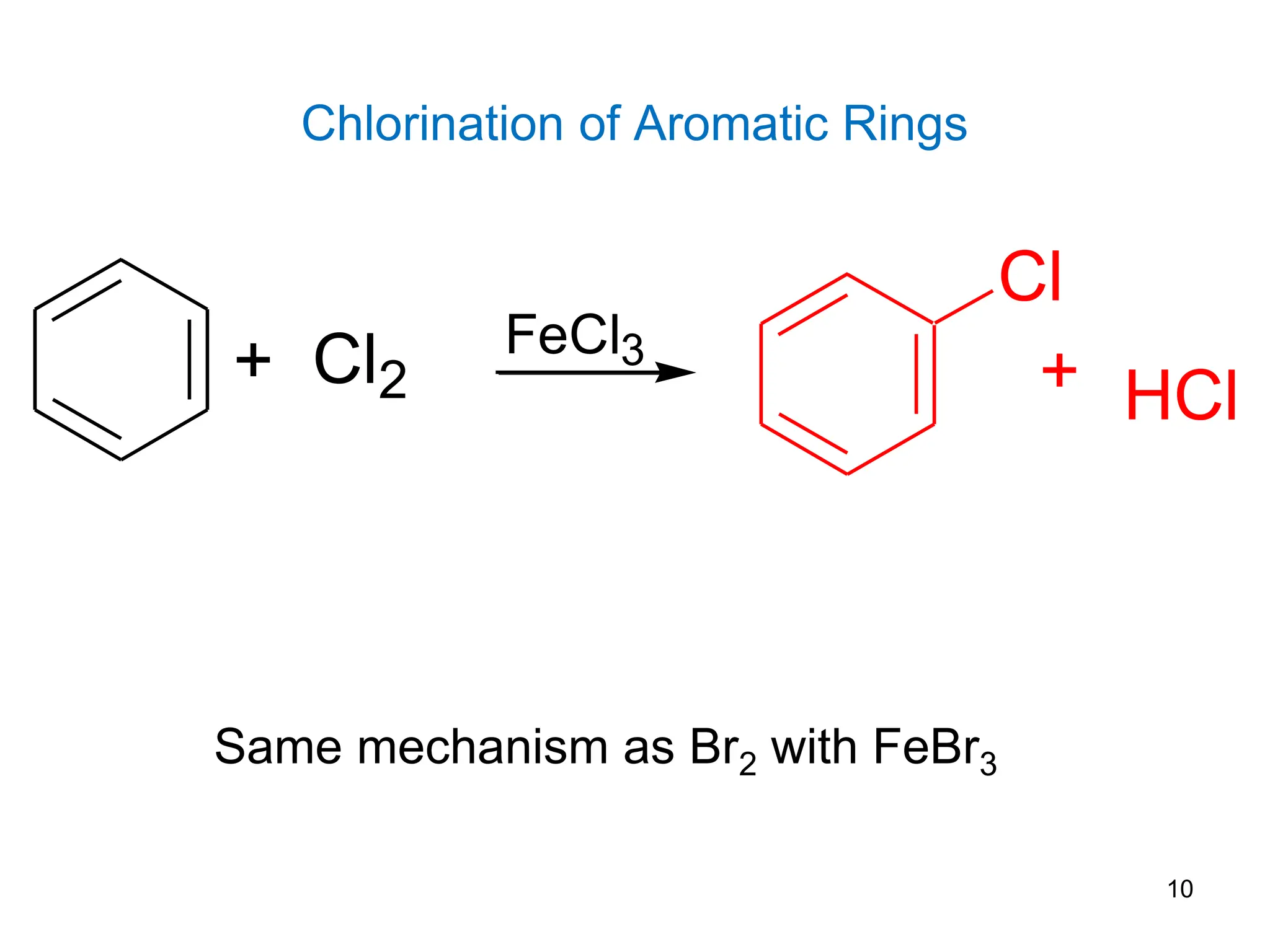
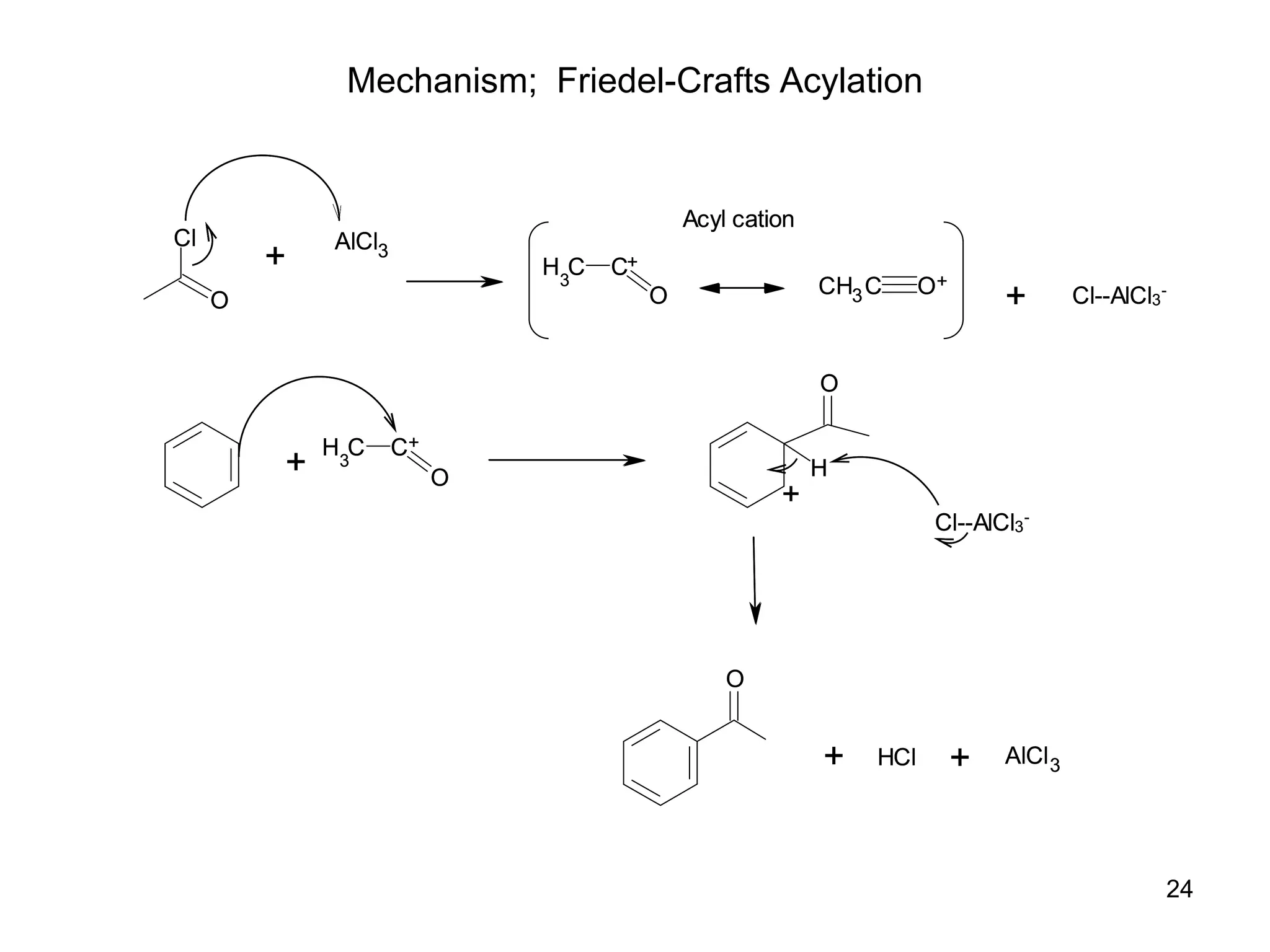
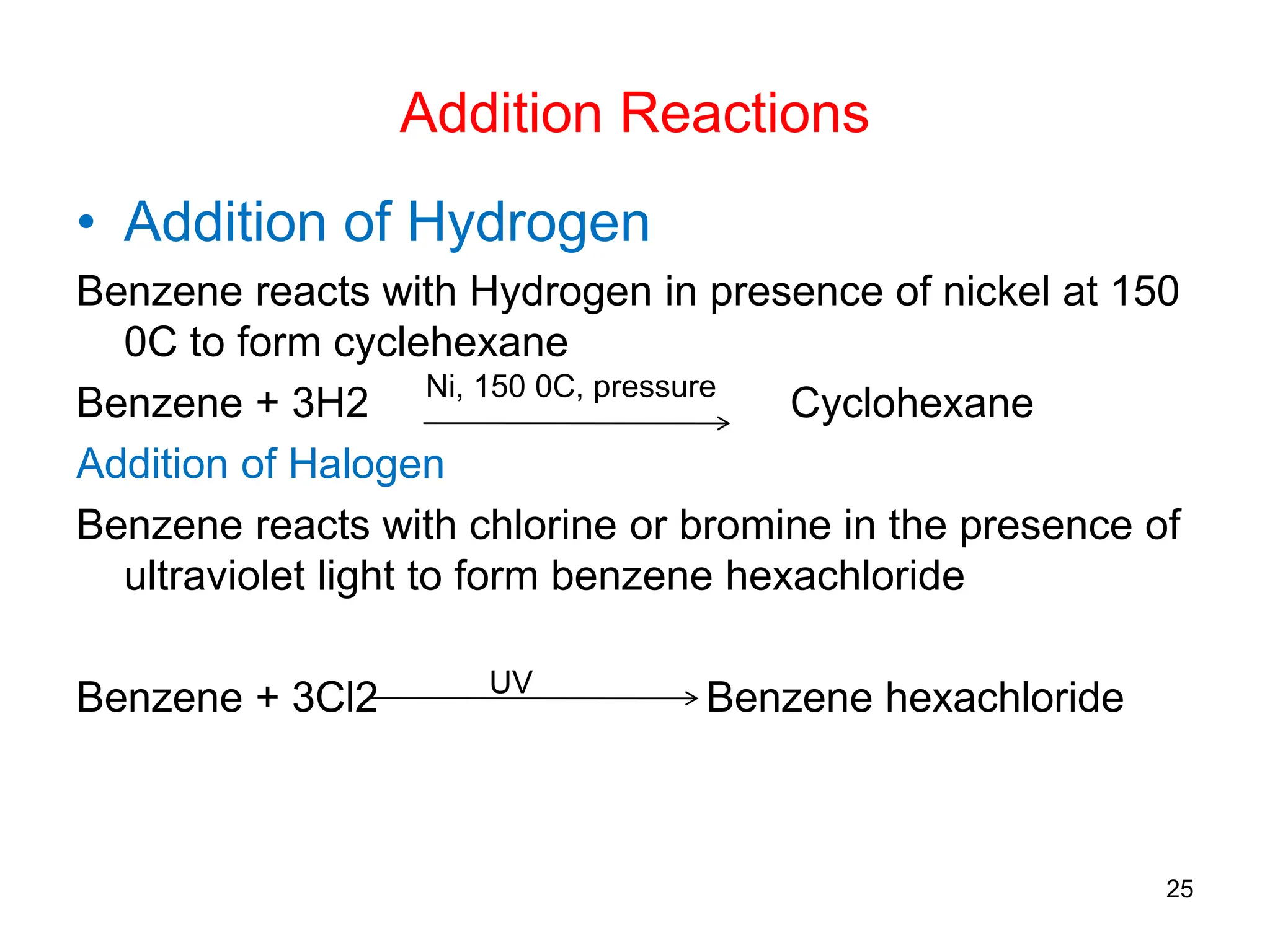
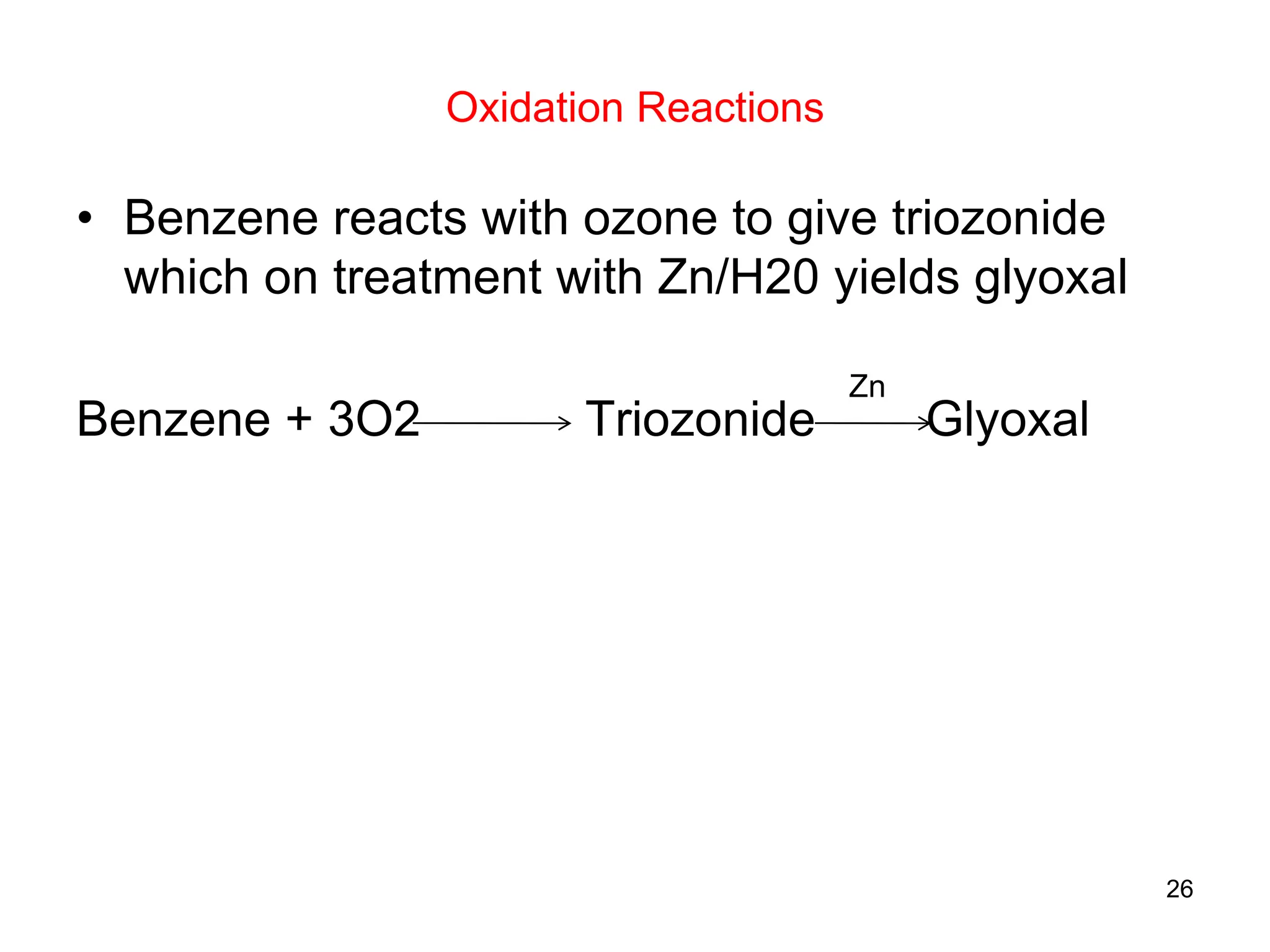
The document discusses various chemical reactions of benzene, focusing on electrophilic substitution, addition, and oxidation reactions. Key processes include halogenation, nitration, sulfonation, Friedman-Crafts reactions, and the conditions under which they occur, such as the necessity of Lewis acids and specific reactive agents. It highlights the mechanisms and products of these reactions while also noting limitations and rearrangements involved in substitution reactions.