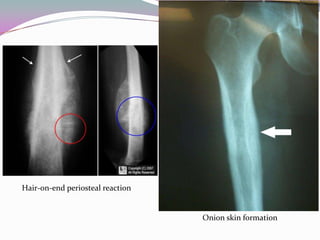
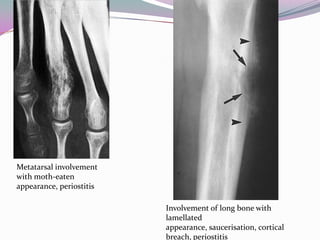
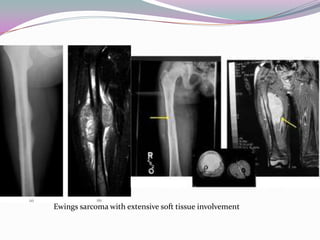
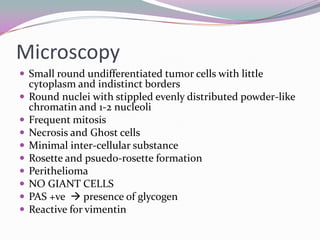
1) Ewing's sarcoma is a rare type of cancer that affects bone or soft tissue. It was first described in 1921 and is thought to arise from stem cells or nerve tissue.
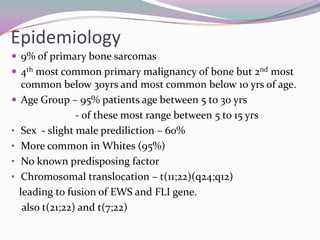
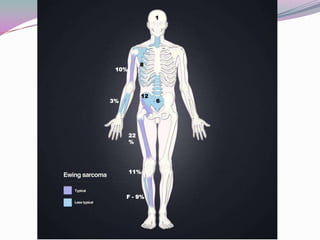
2) It most commonly affects children and young adults between 5-30 years old and presents with pain, swelling, fever, weight loss and anemia. Common sites are the lower limbs, pelvis, ribs, and spine.
3) Treatment involves chemotherapy, surgery, and radiation therapy. The standard chemotherapy regimen is VAC or IE. Prognosis is best if the cancer is localized and in younger patients, and worst if it has spread to other organs or the patient is older.