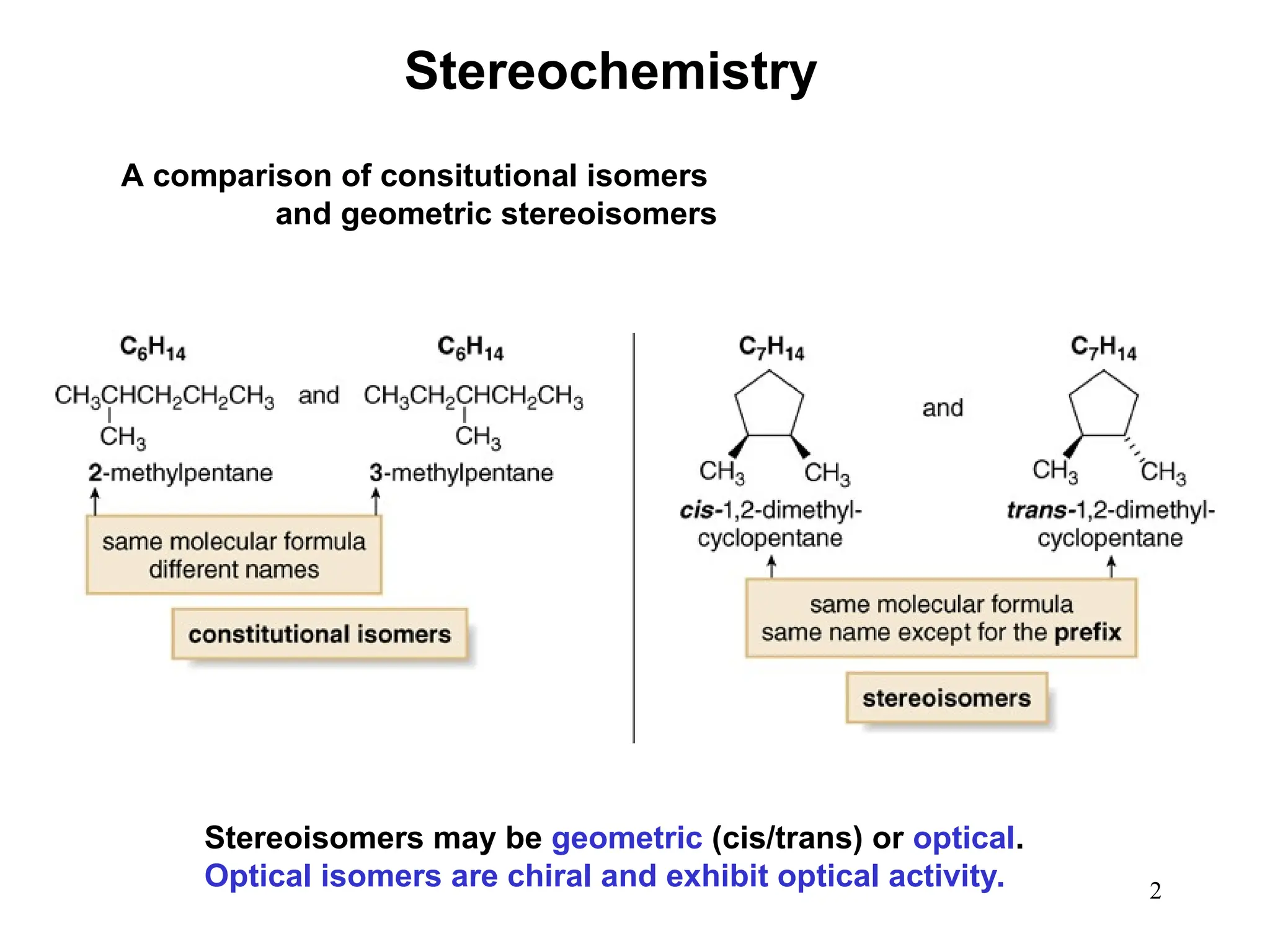
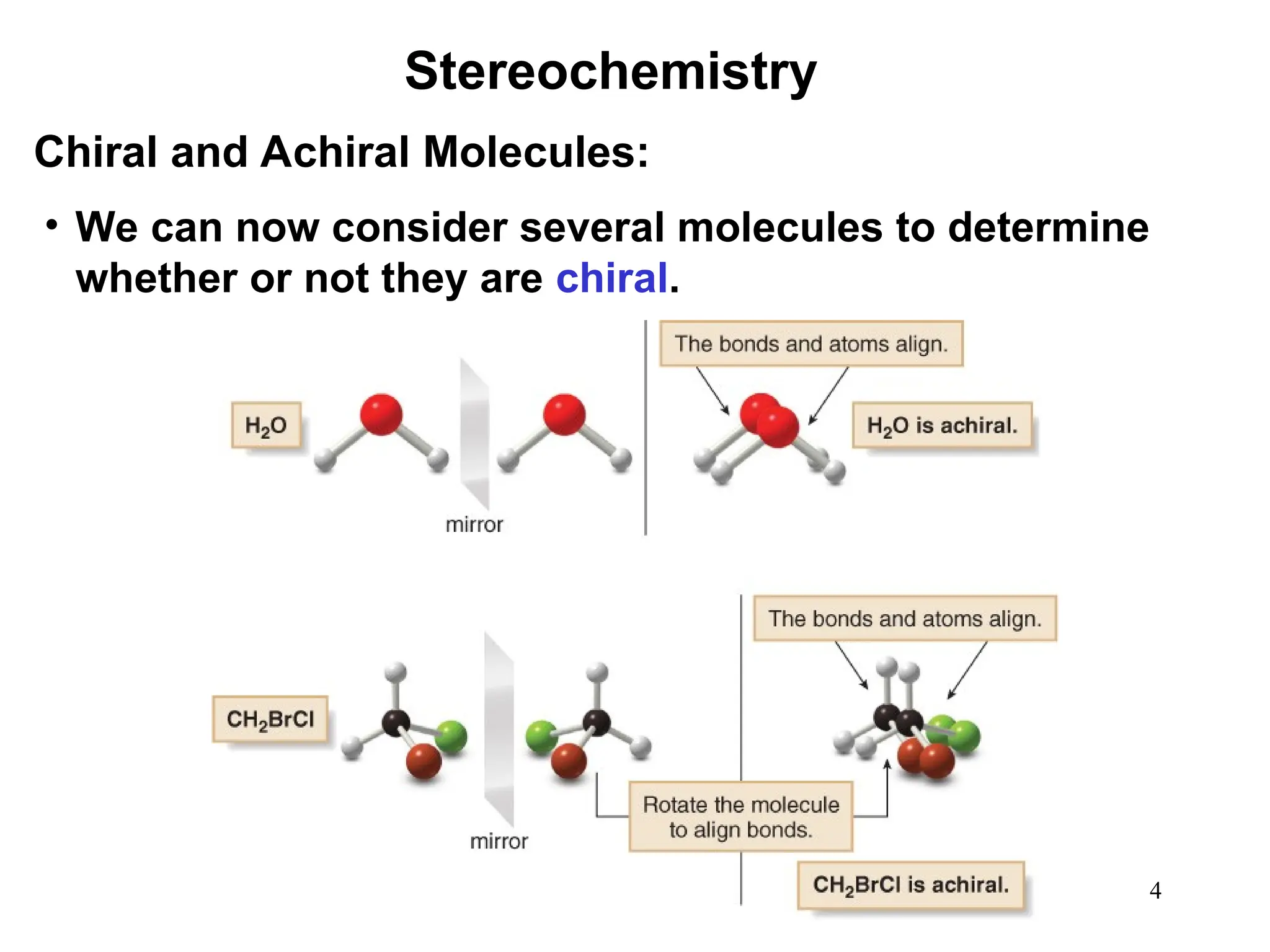
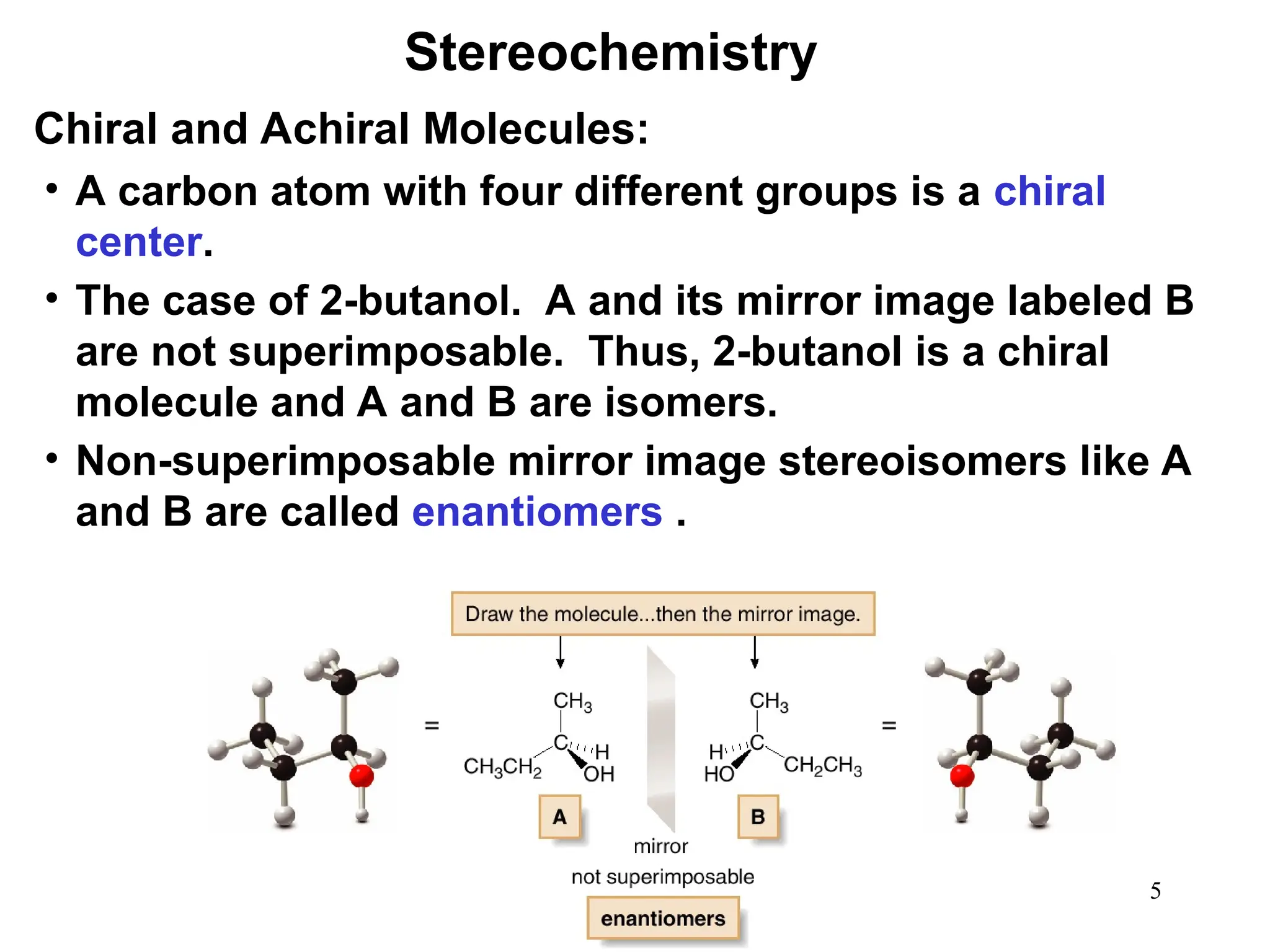
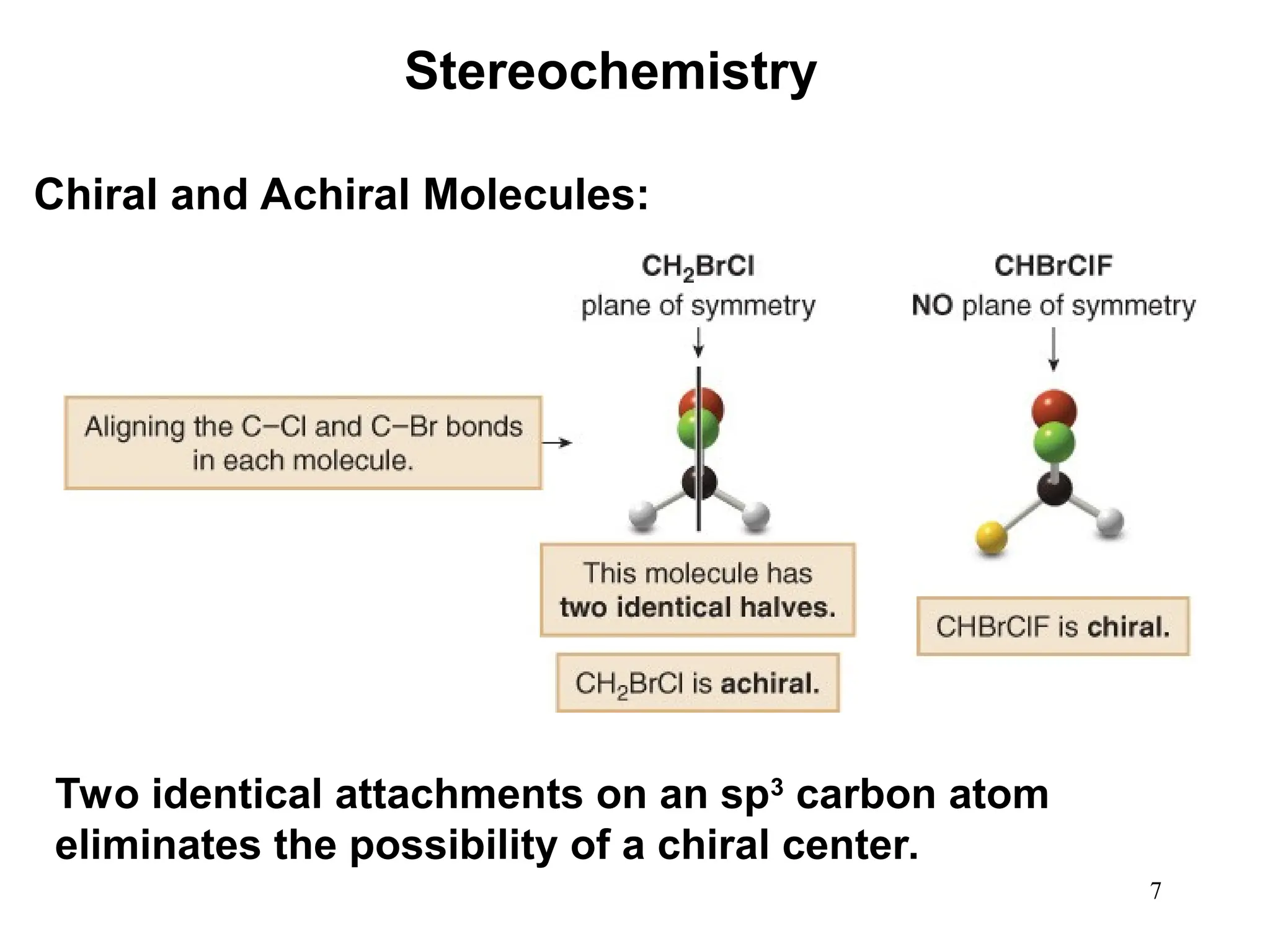
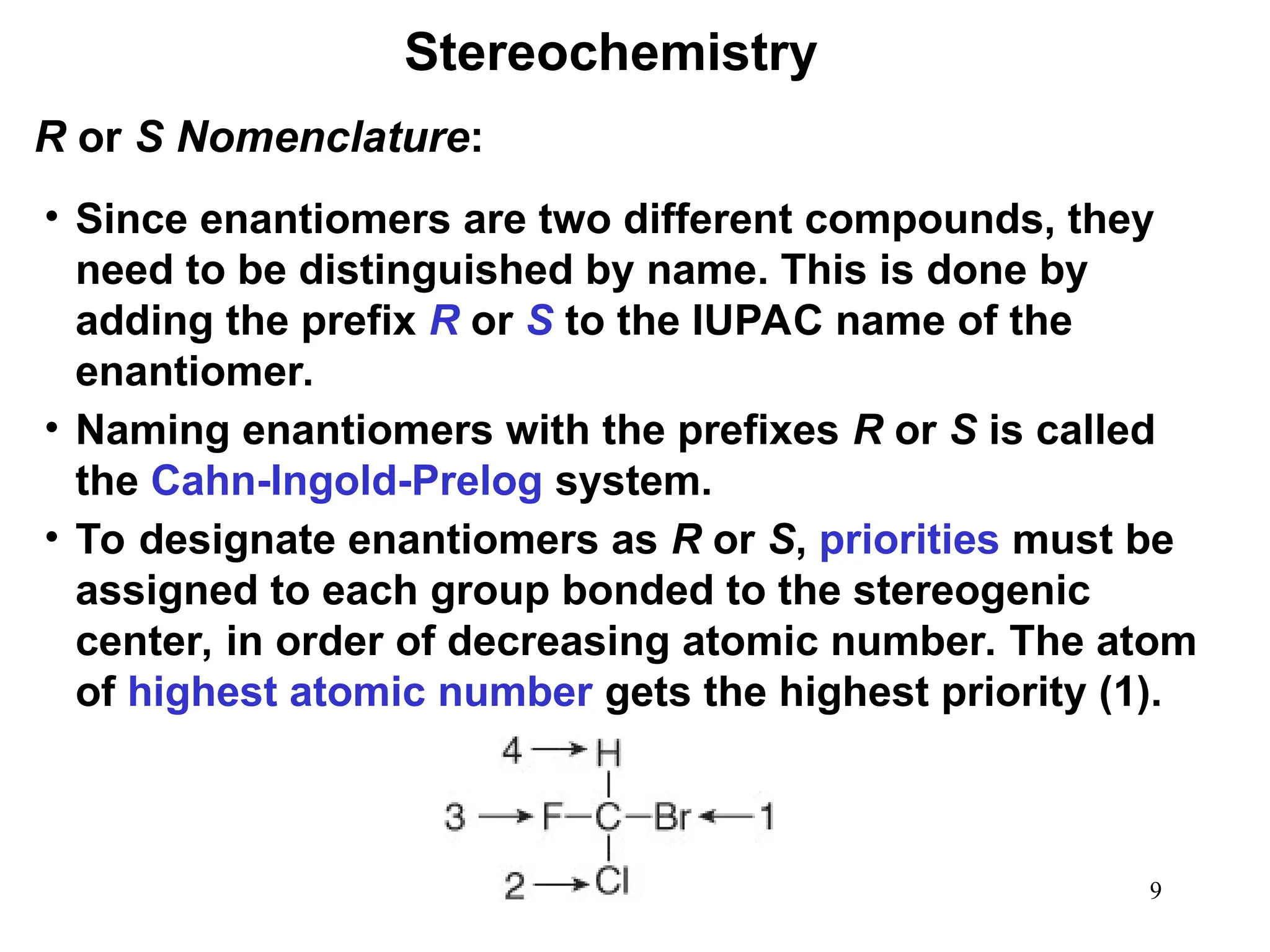
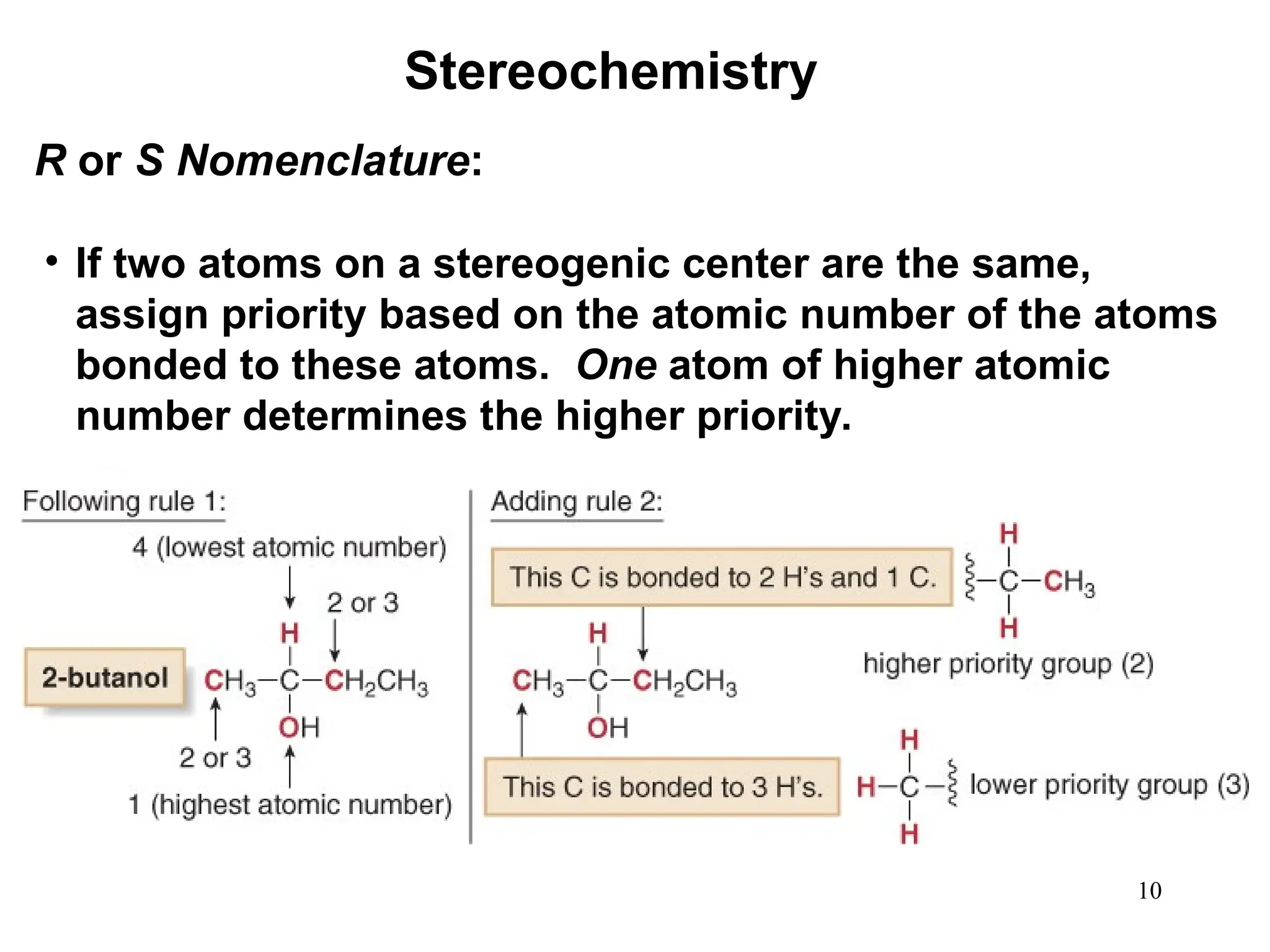
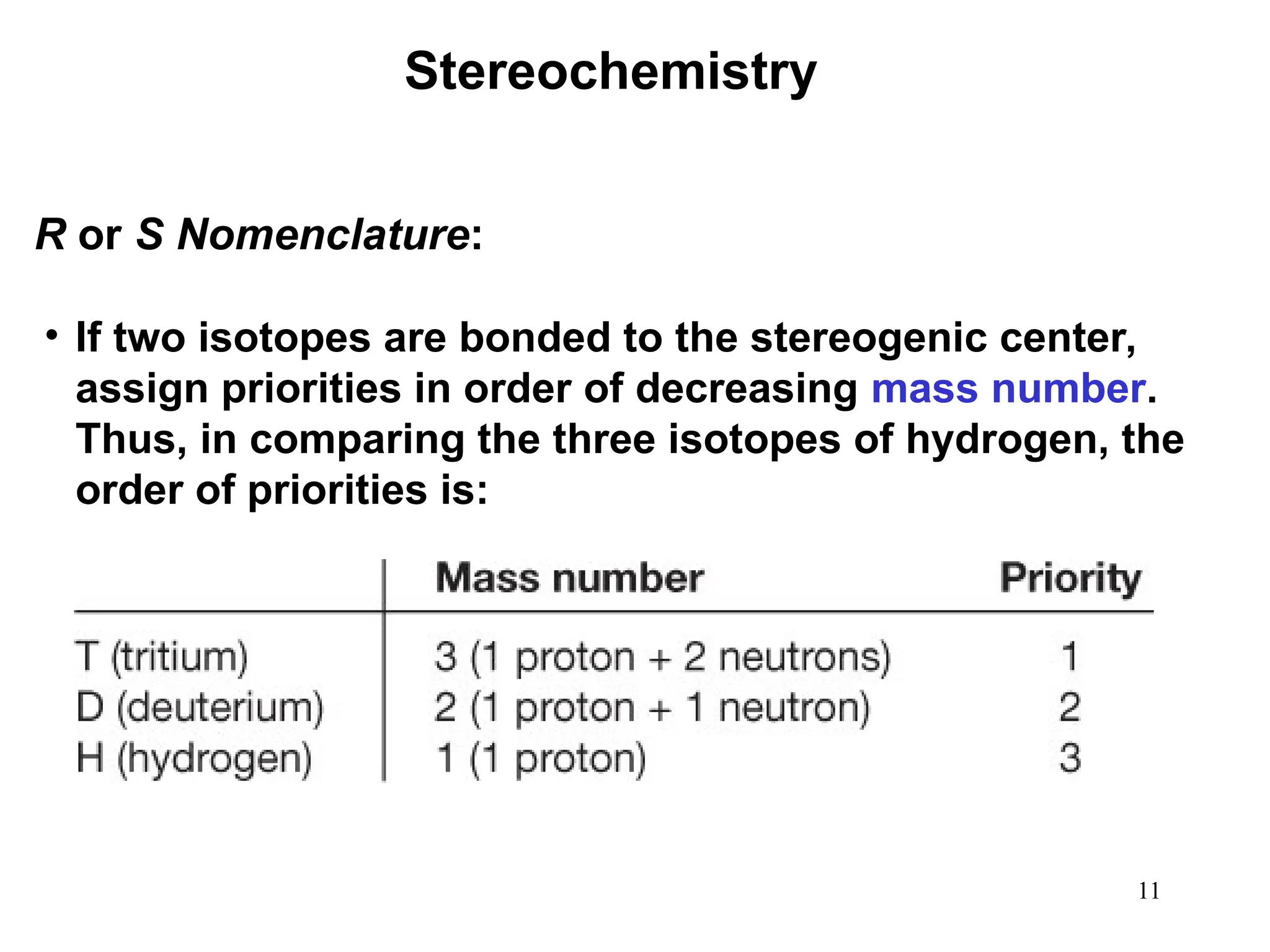
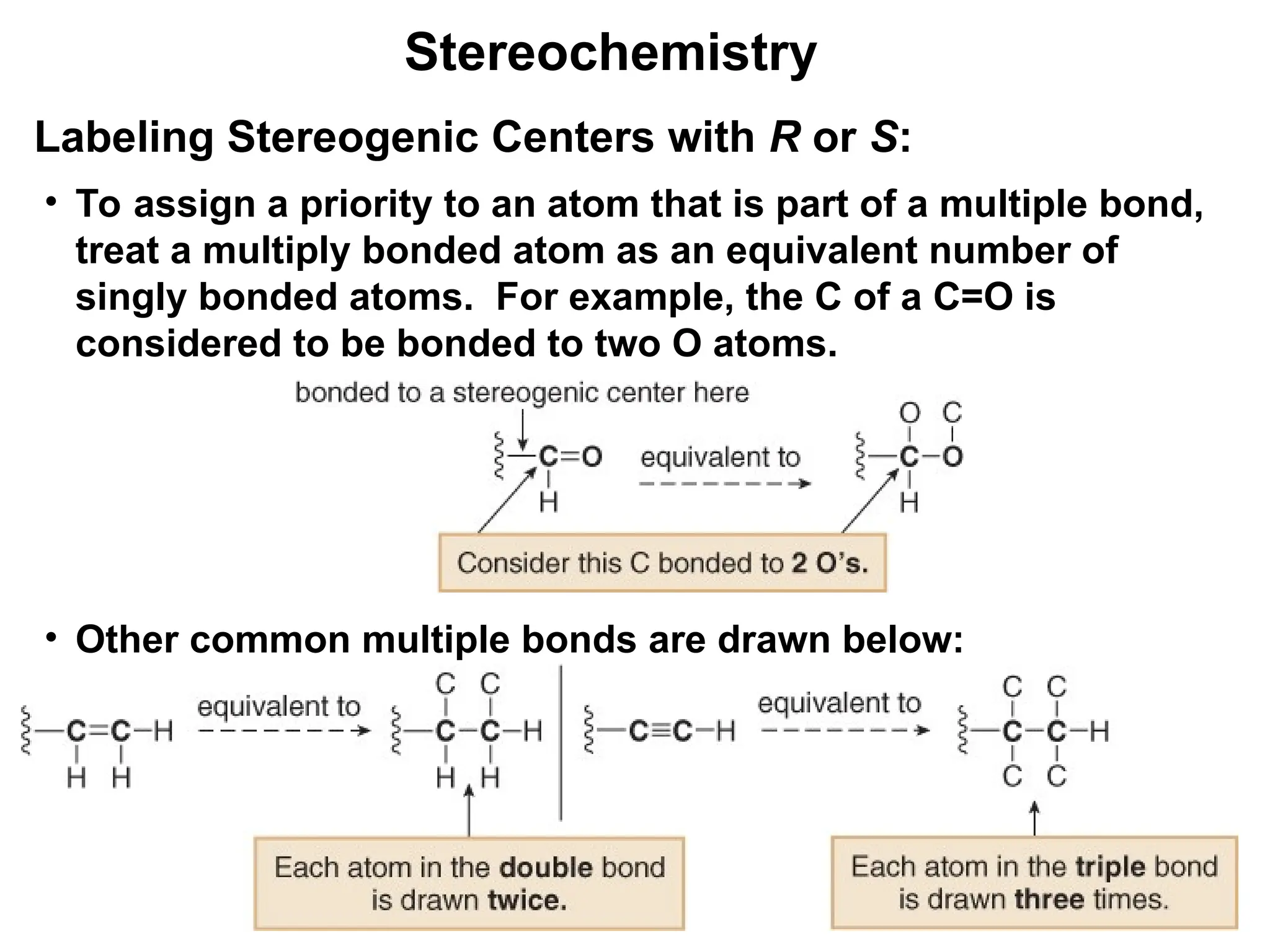
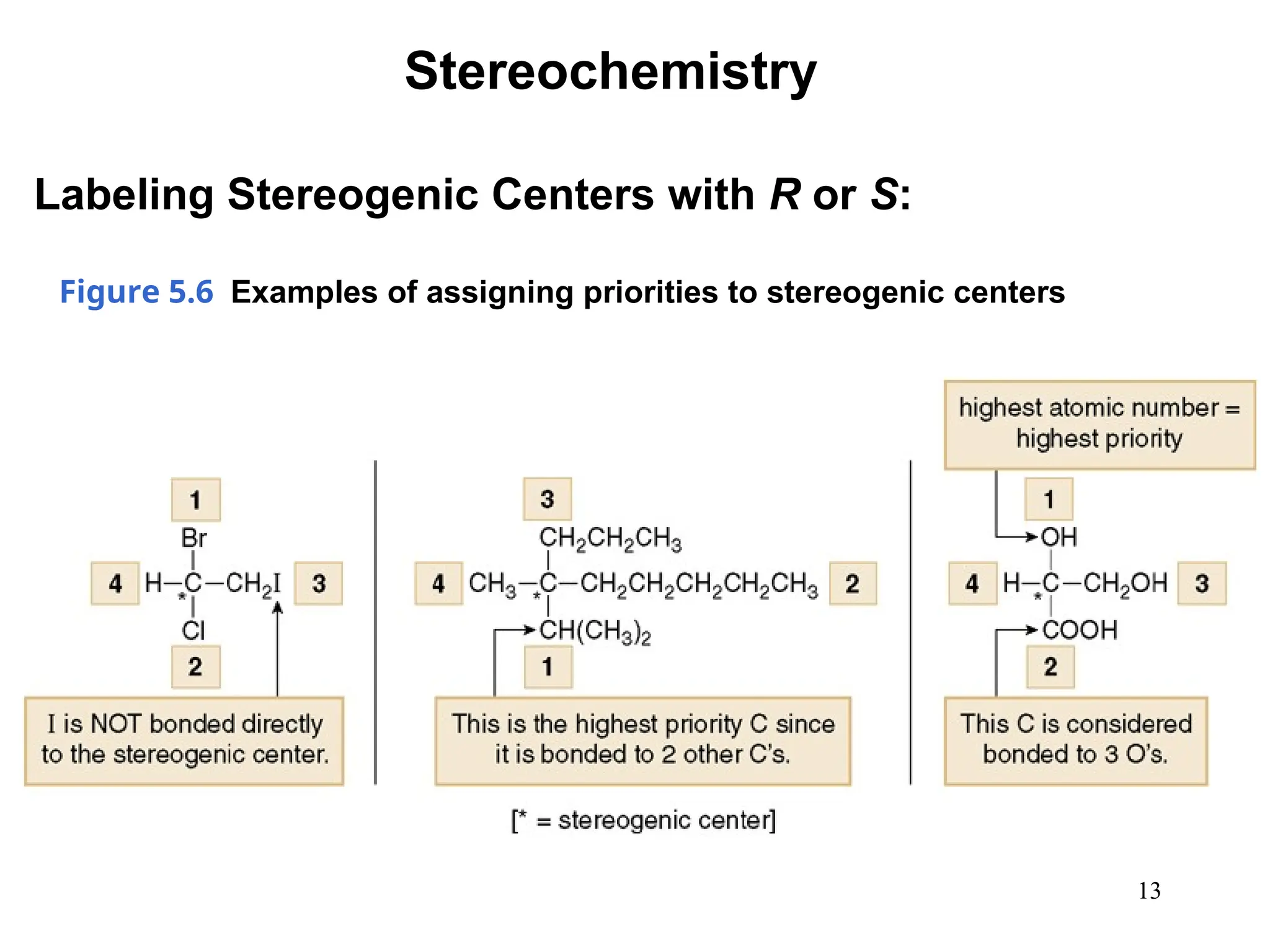
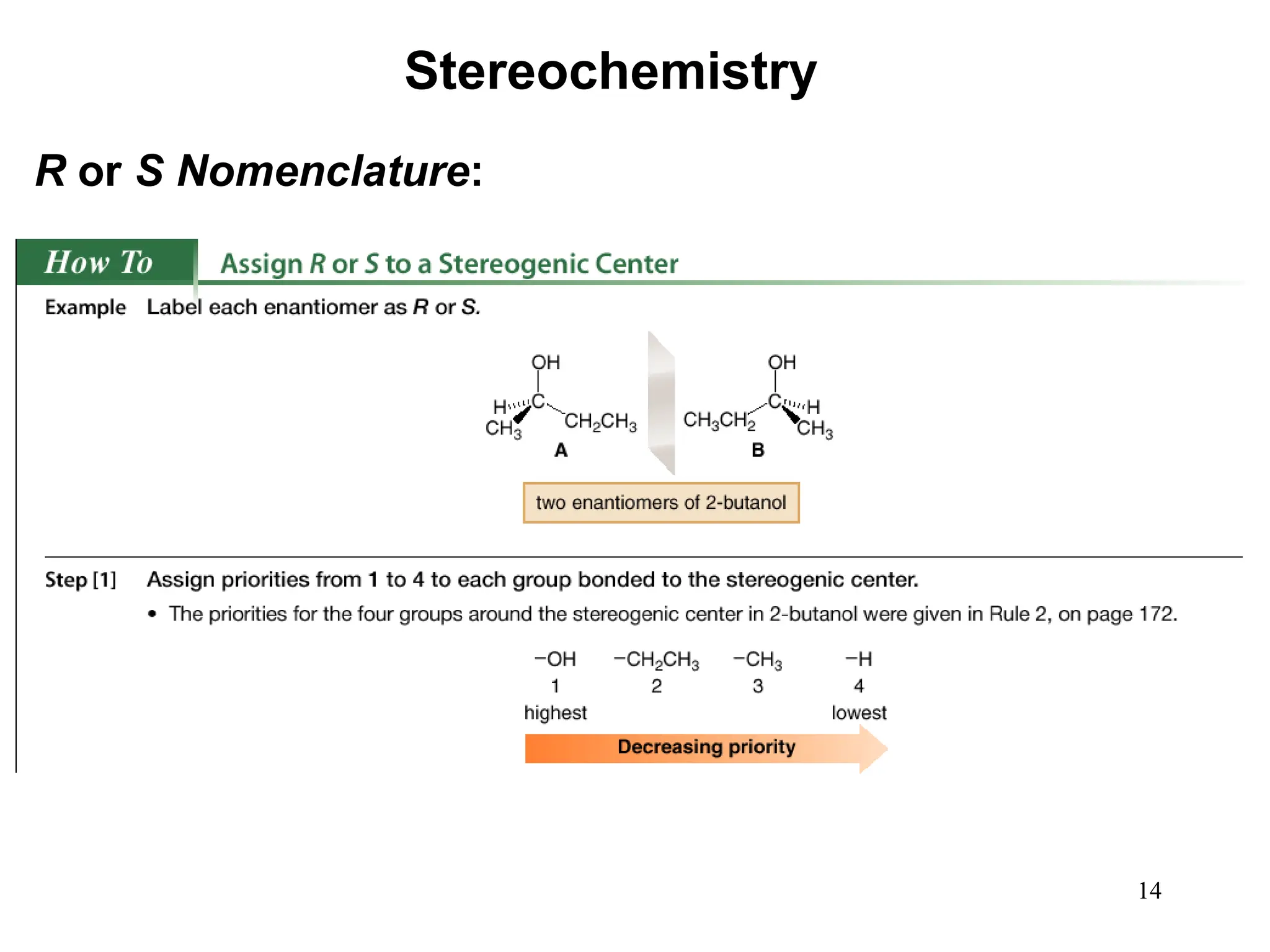
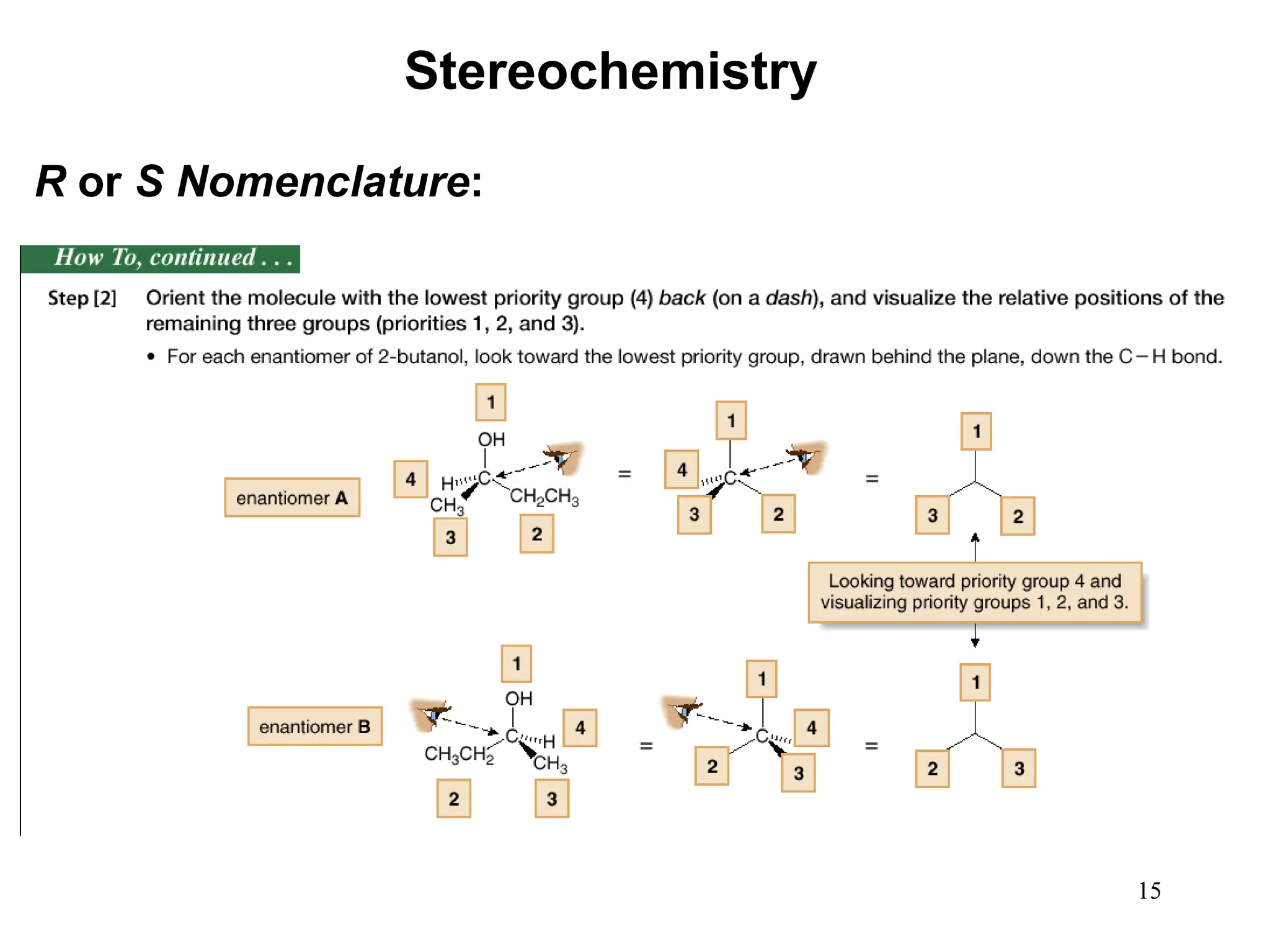
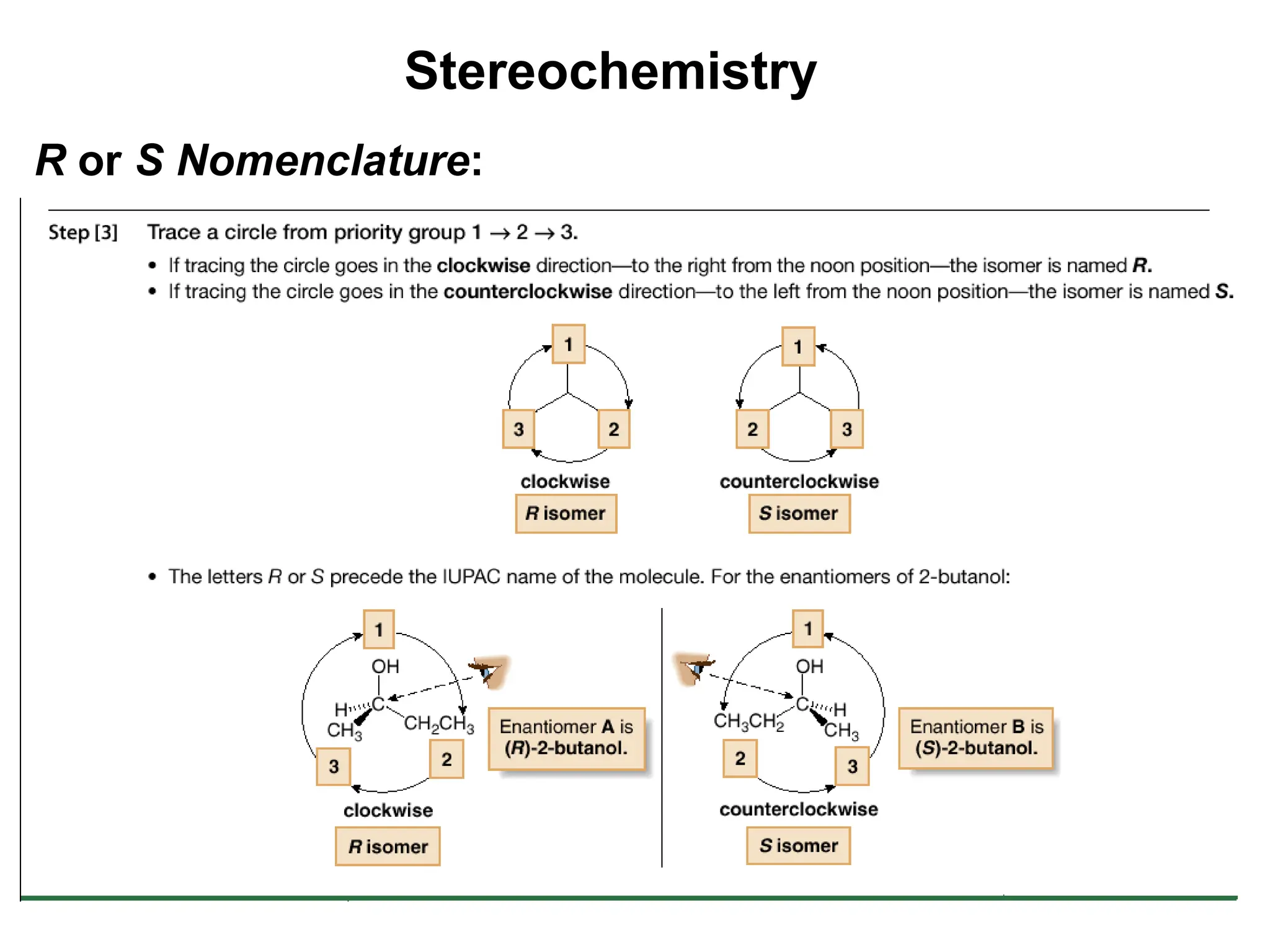
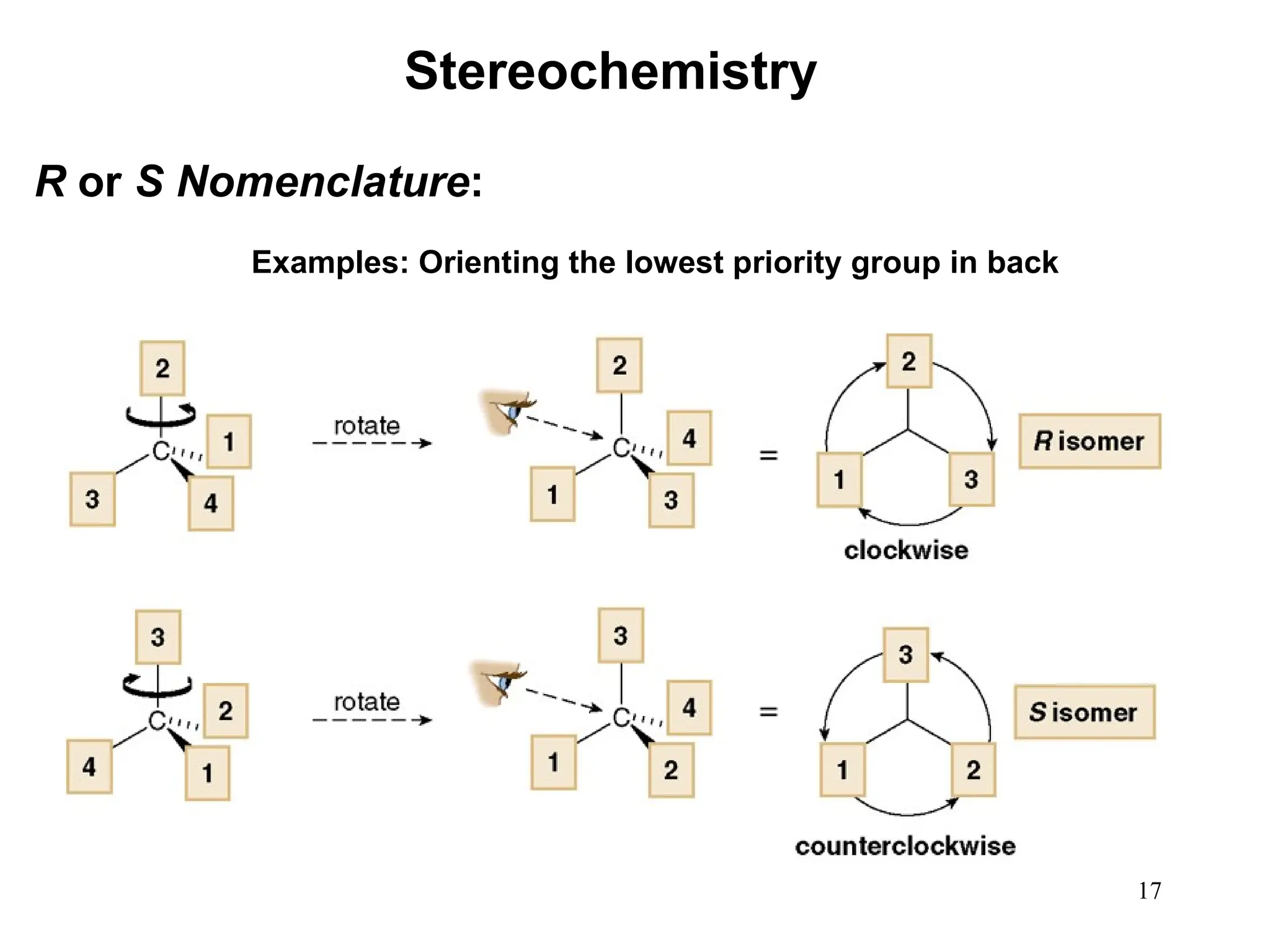
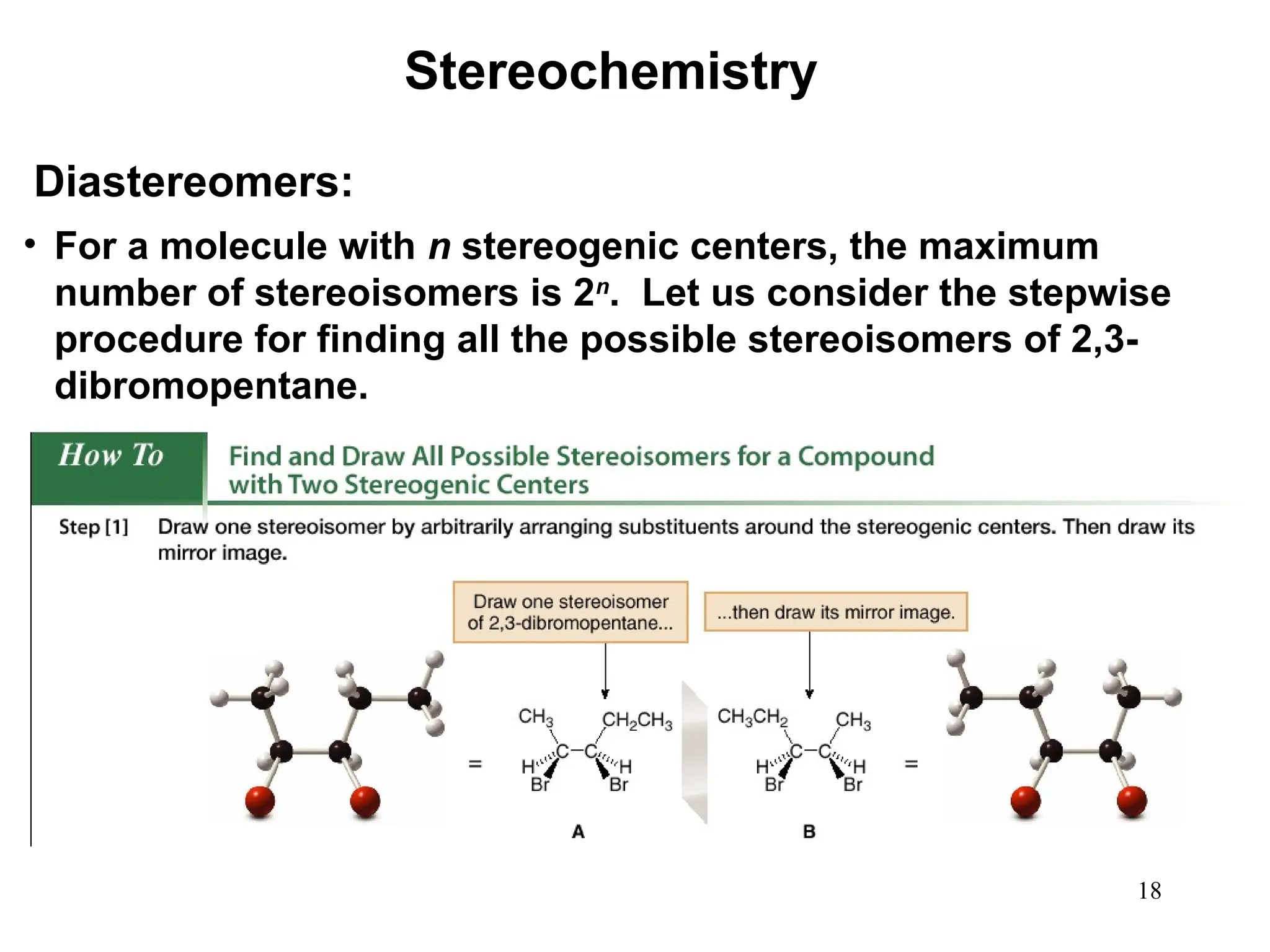
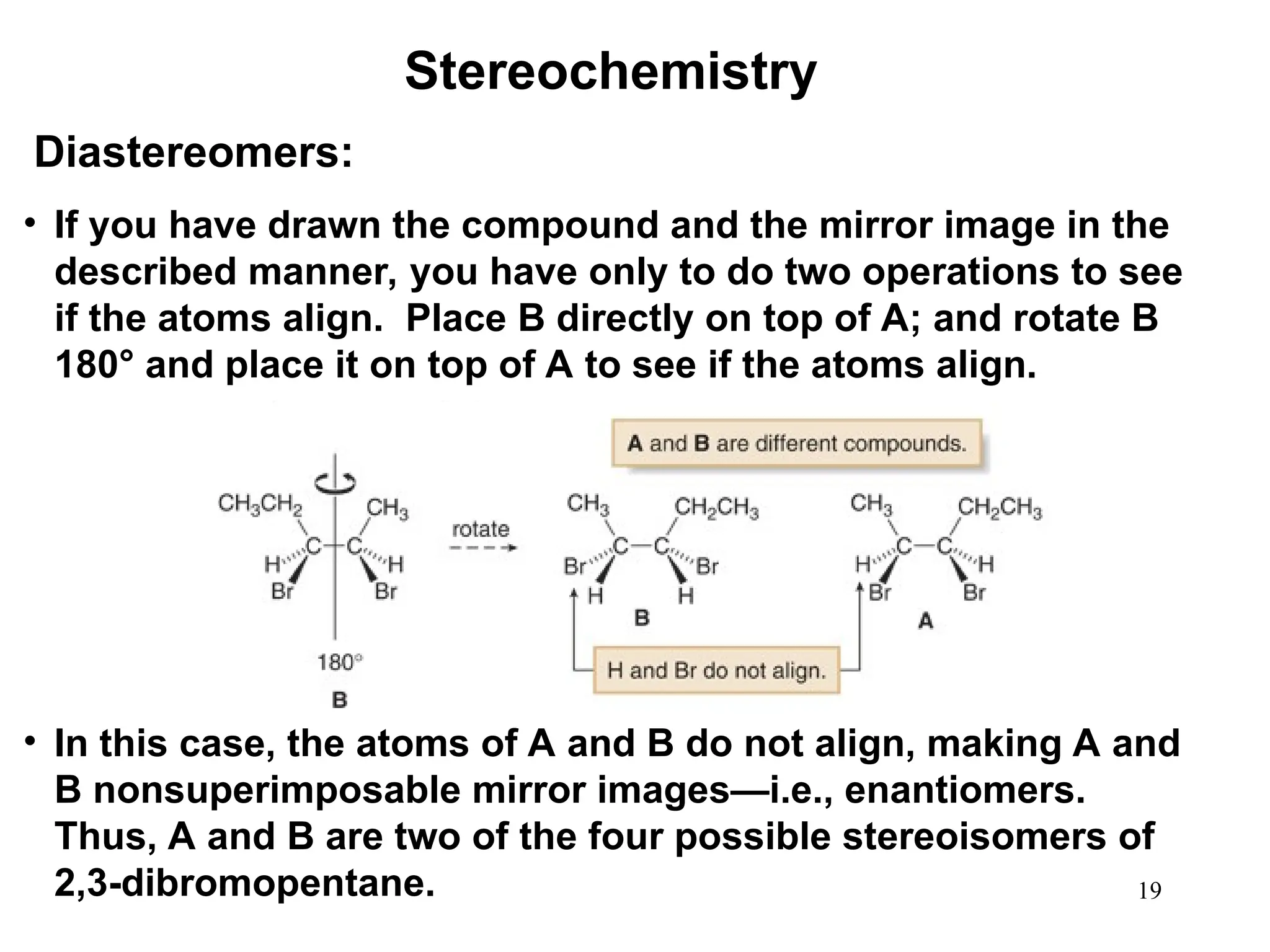
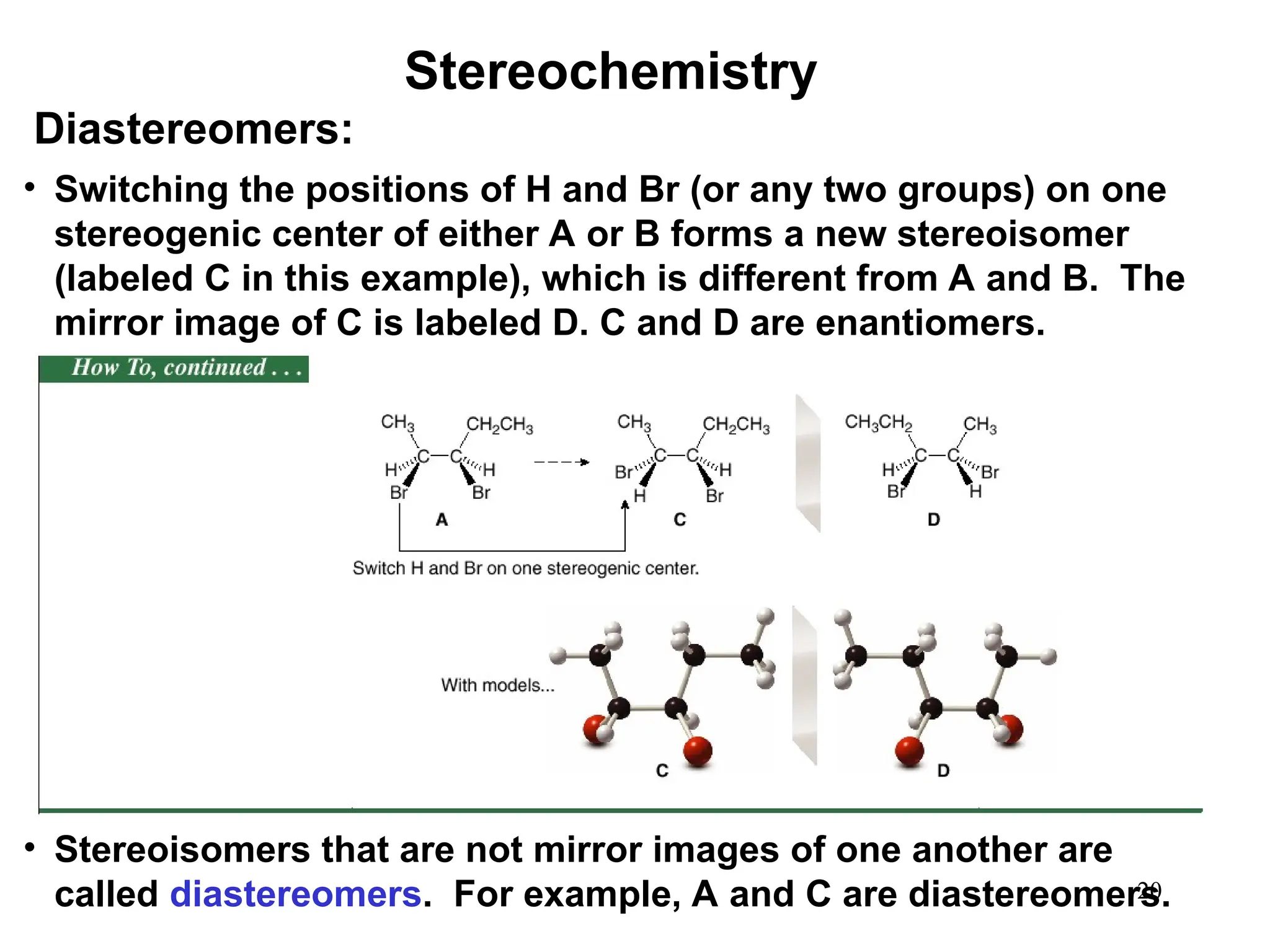
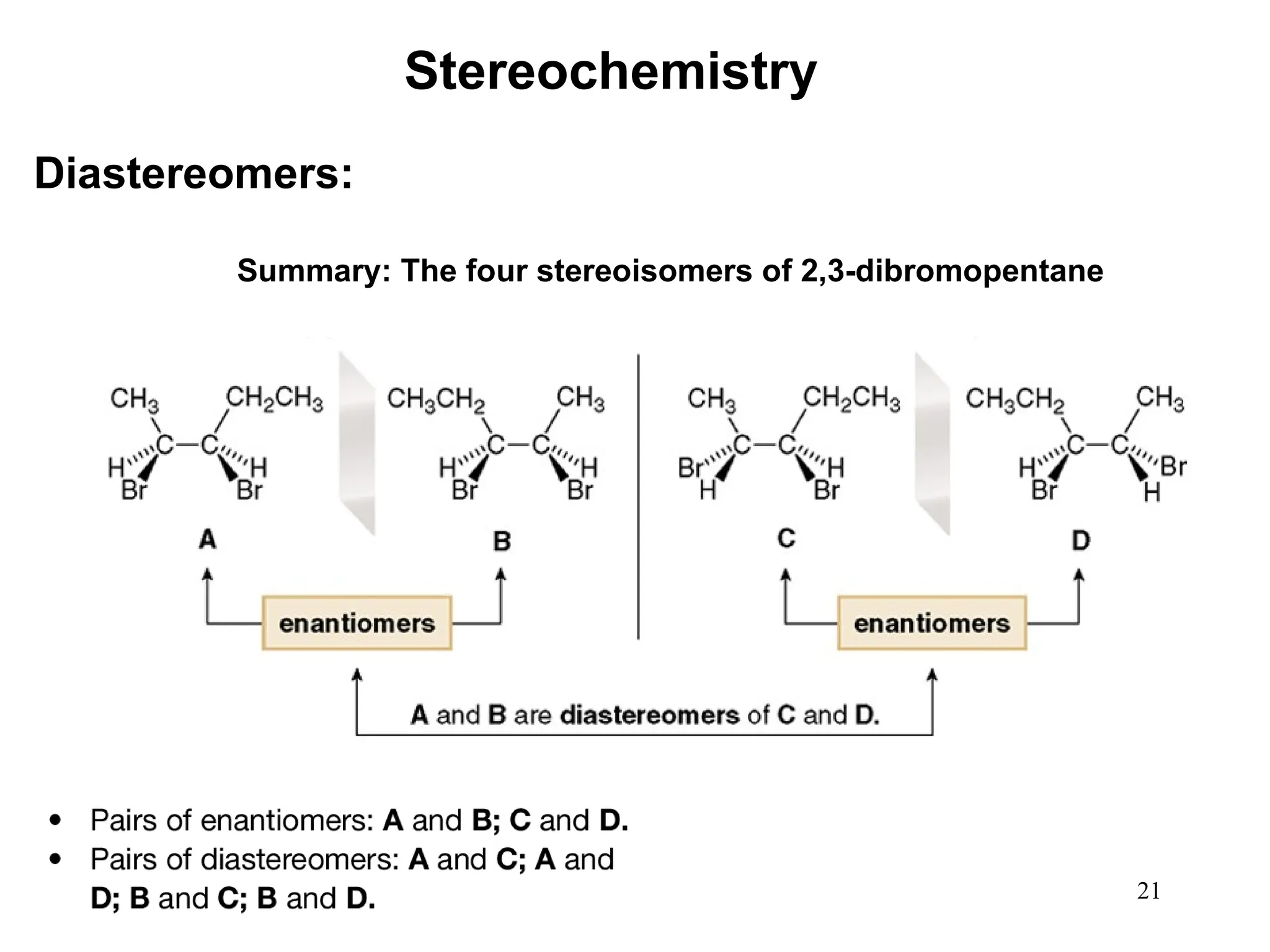
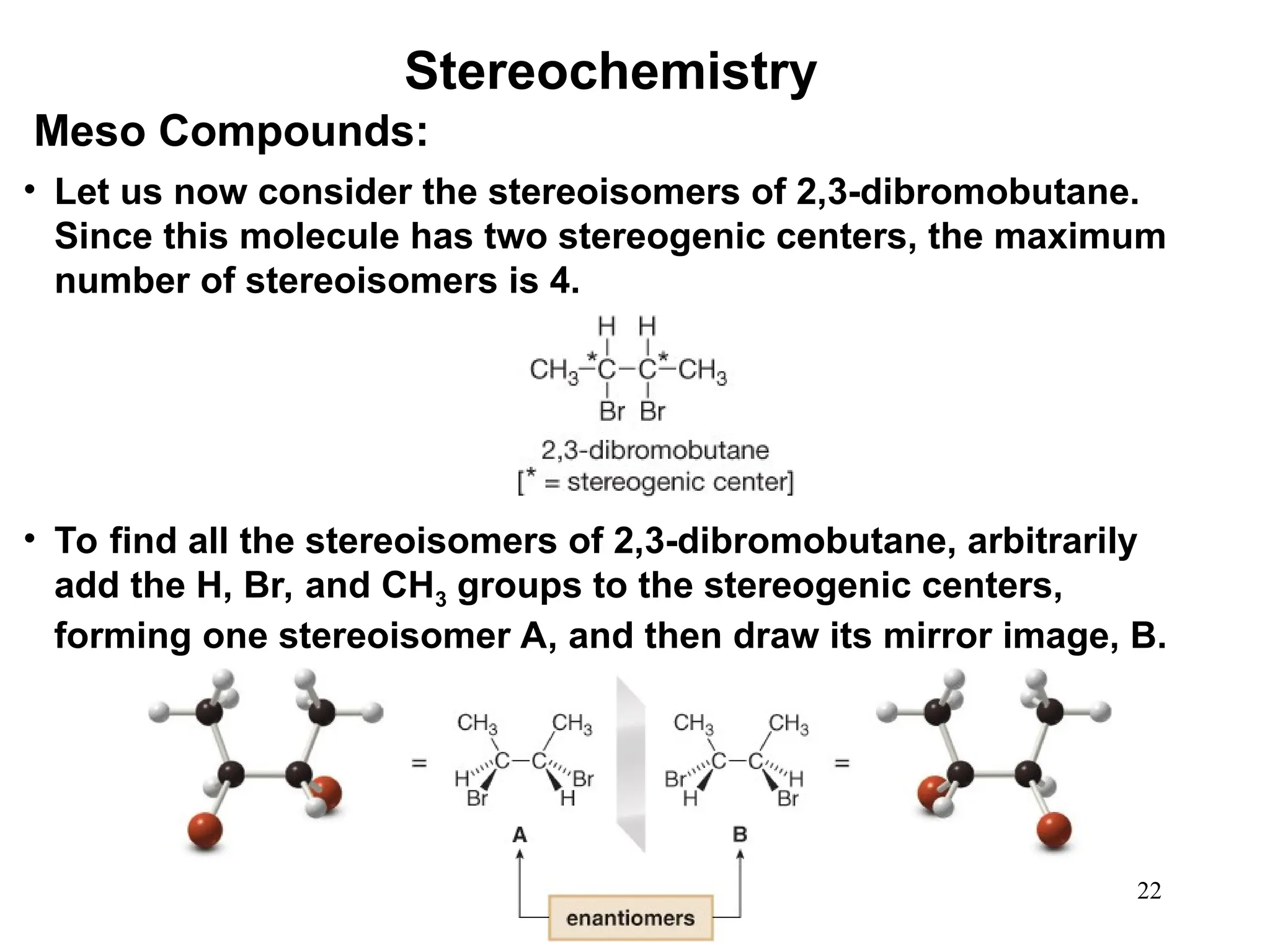
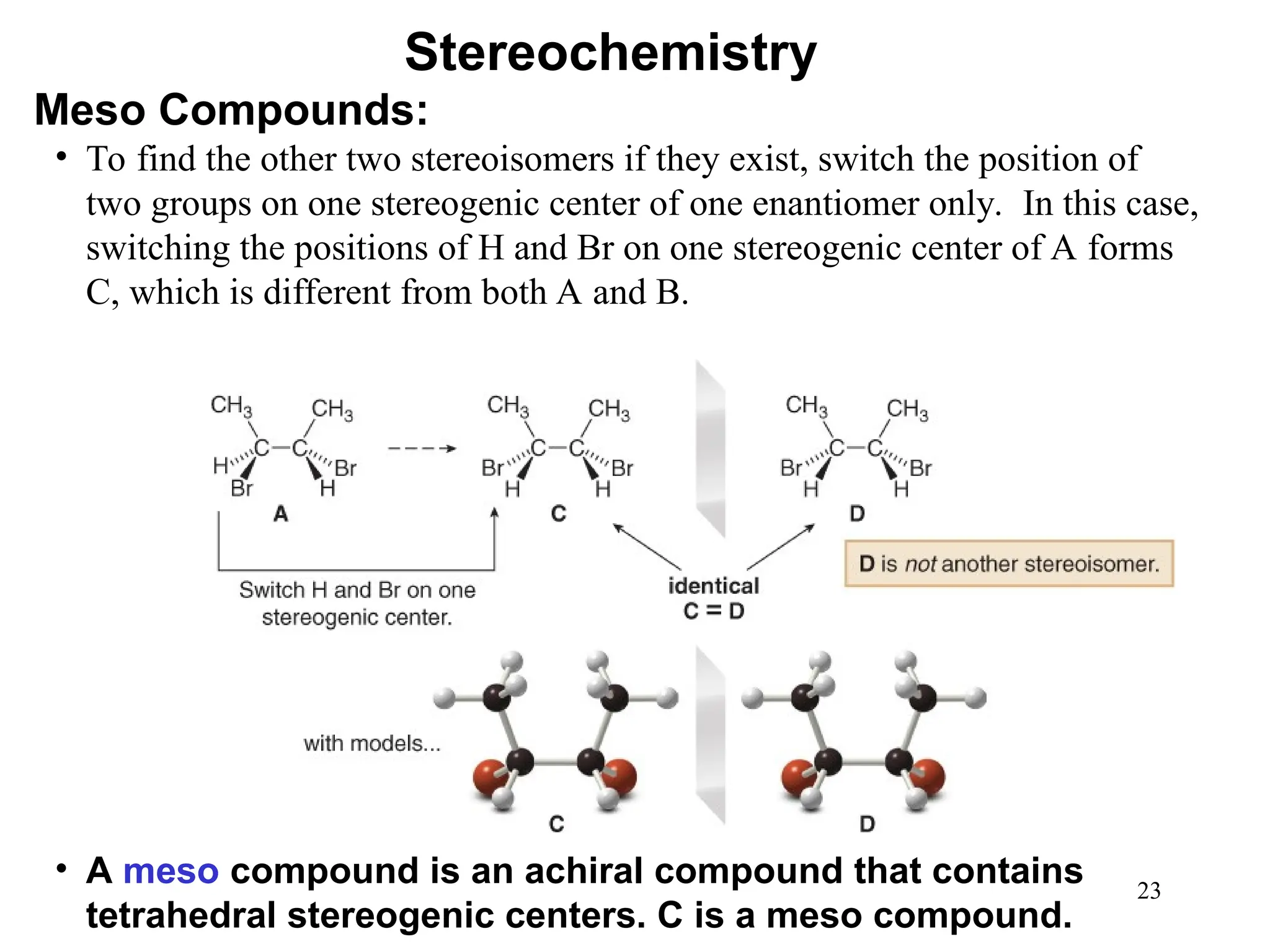
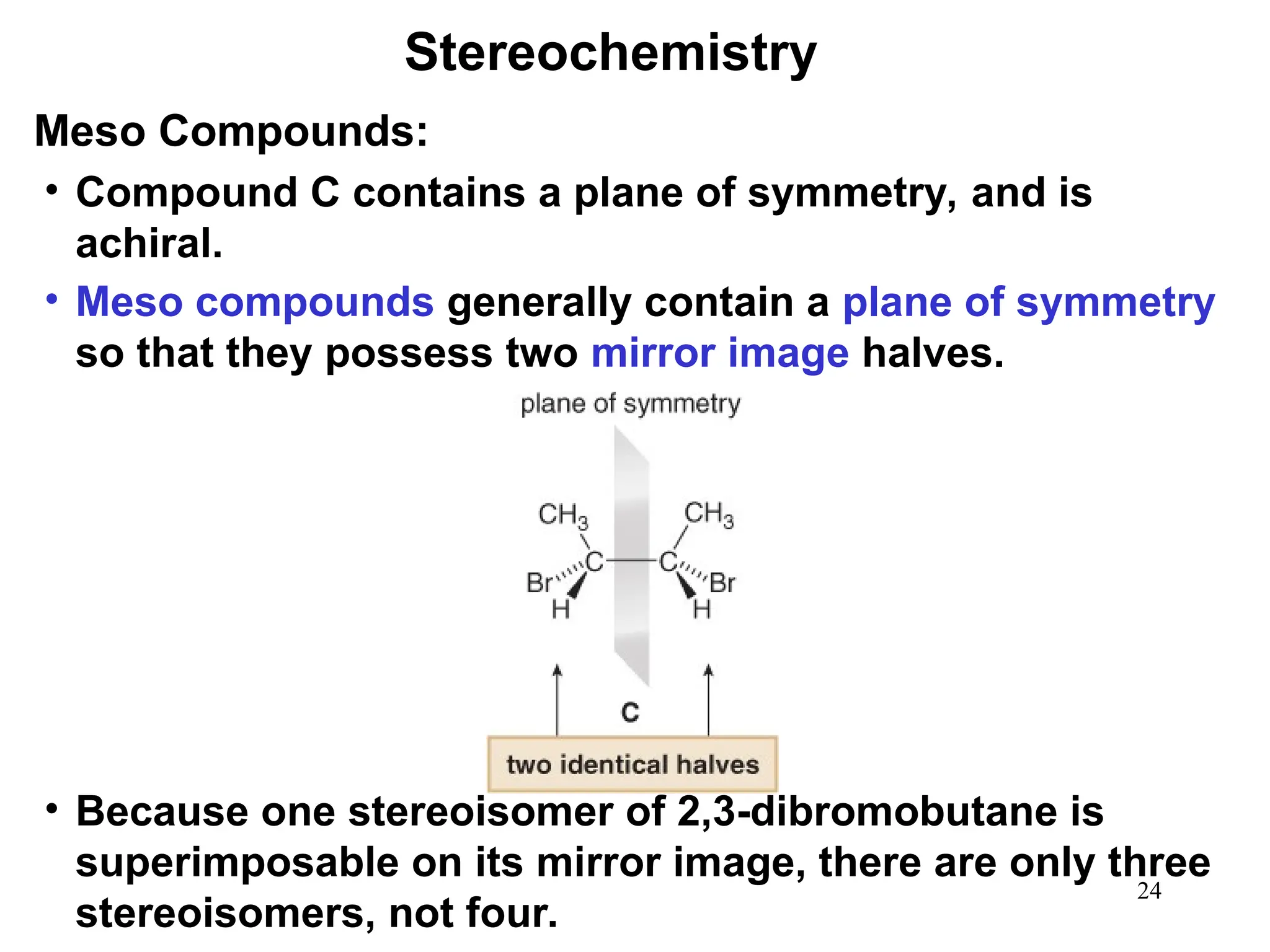
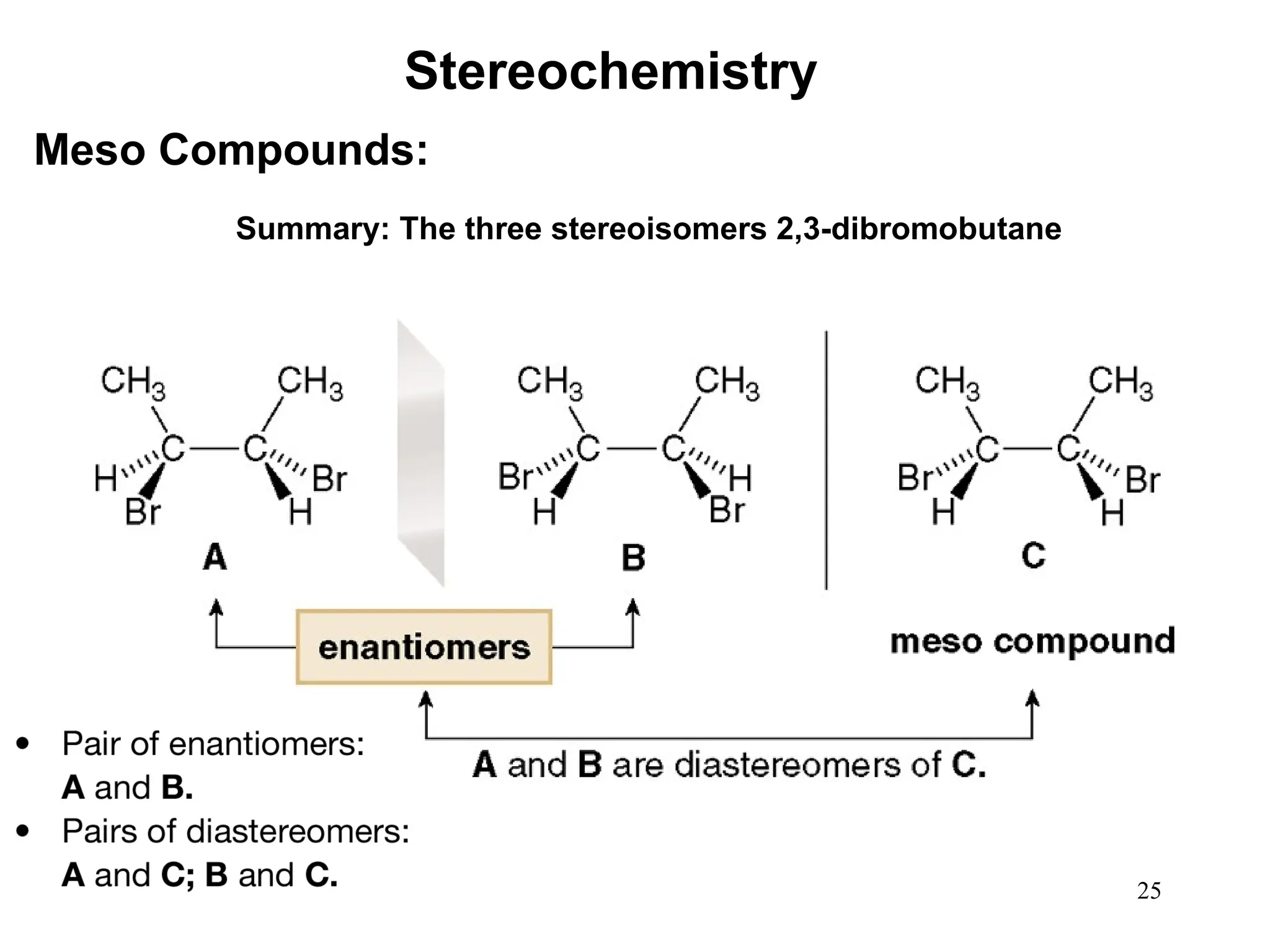
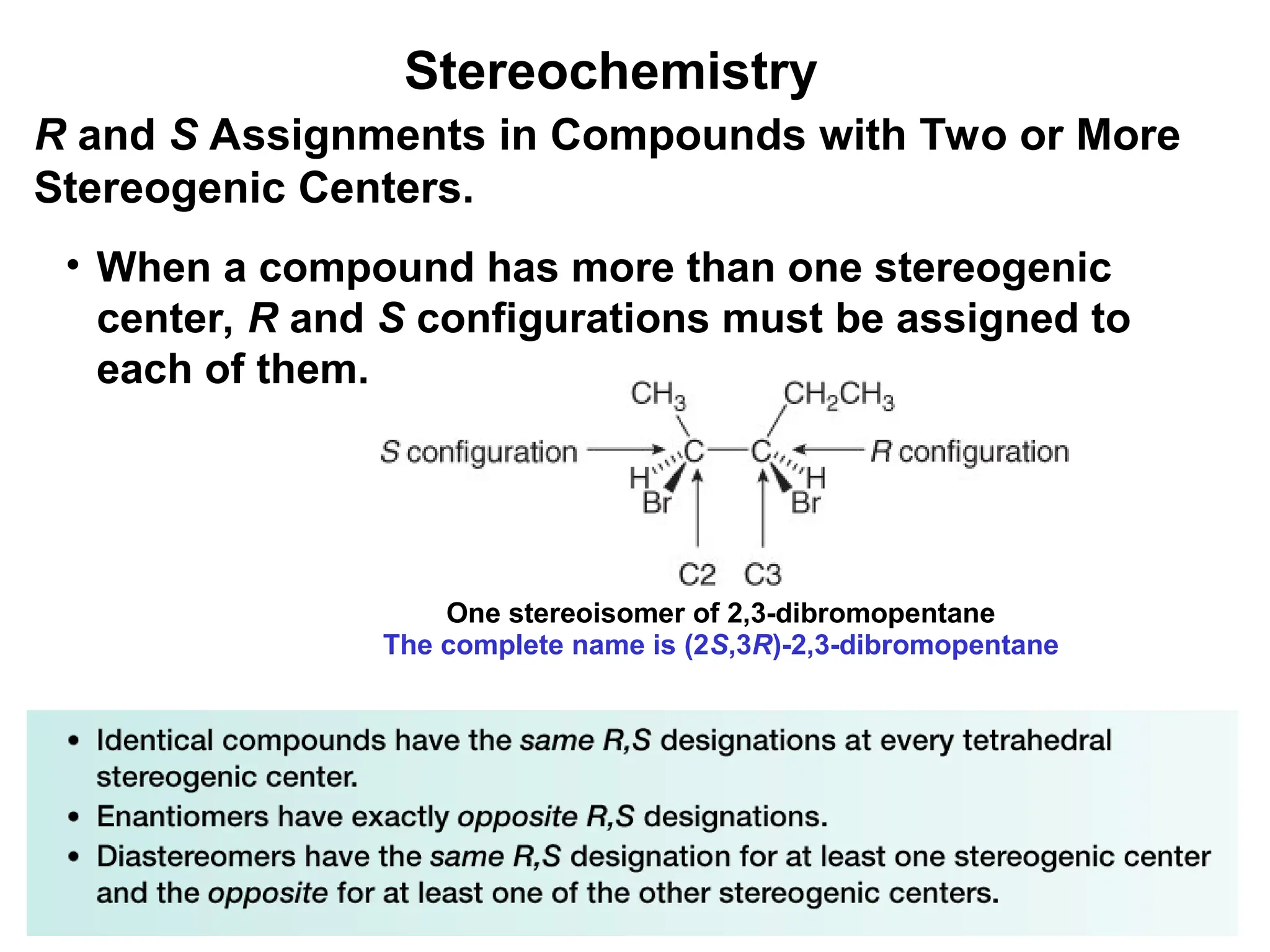
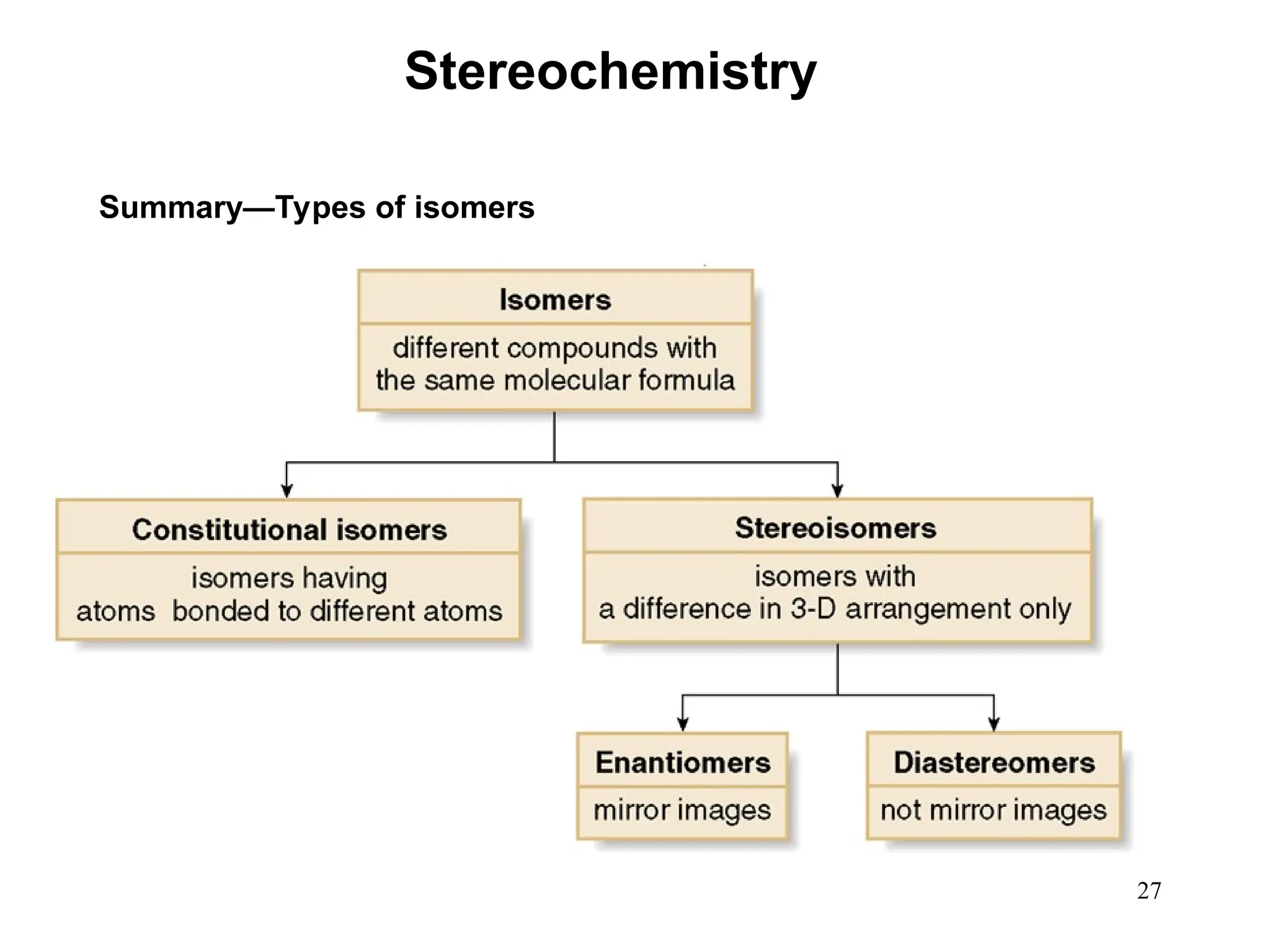
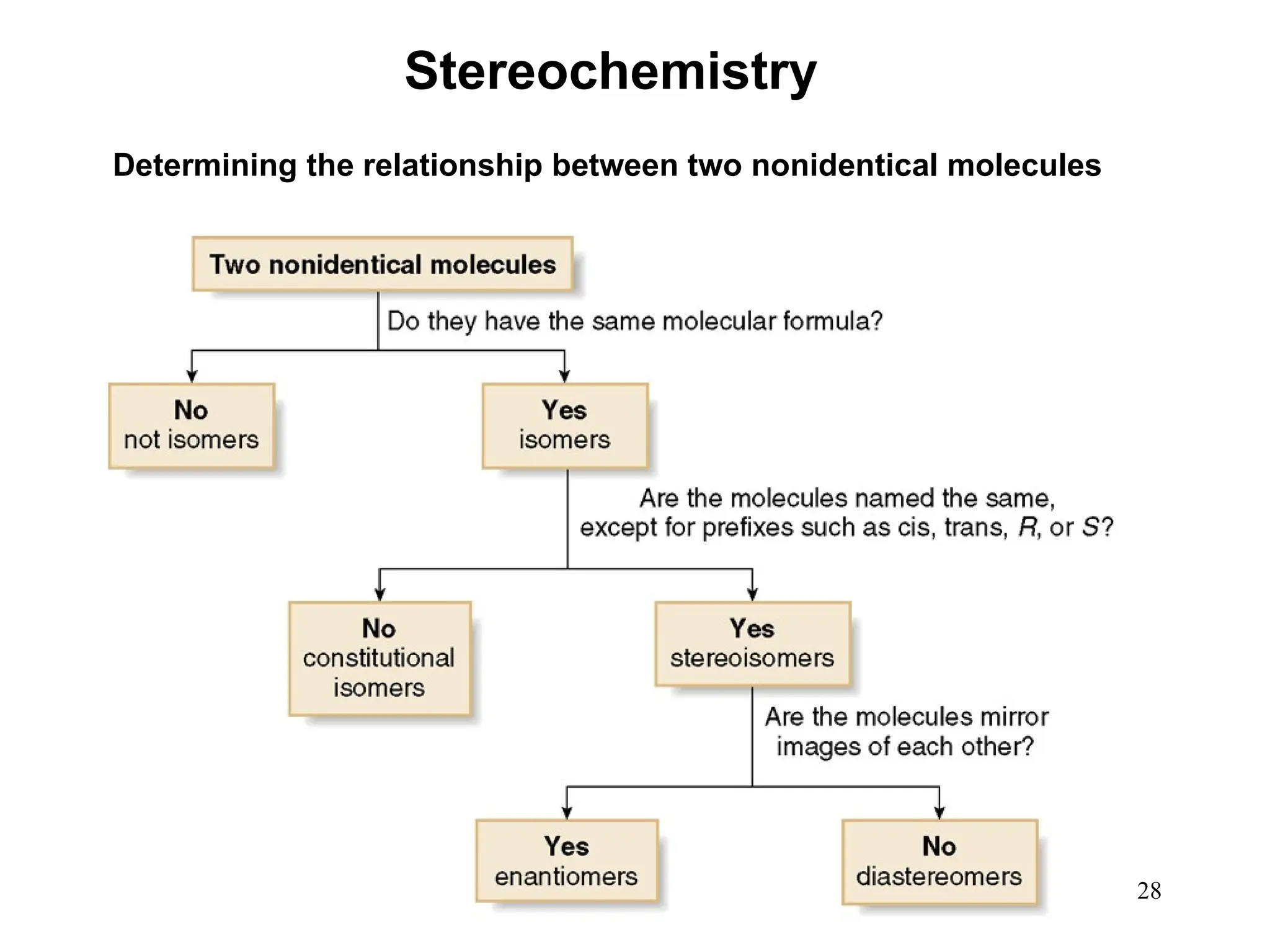
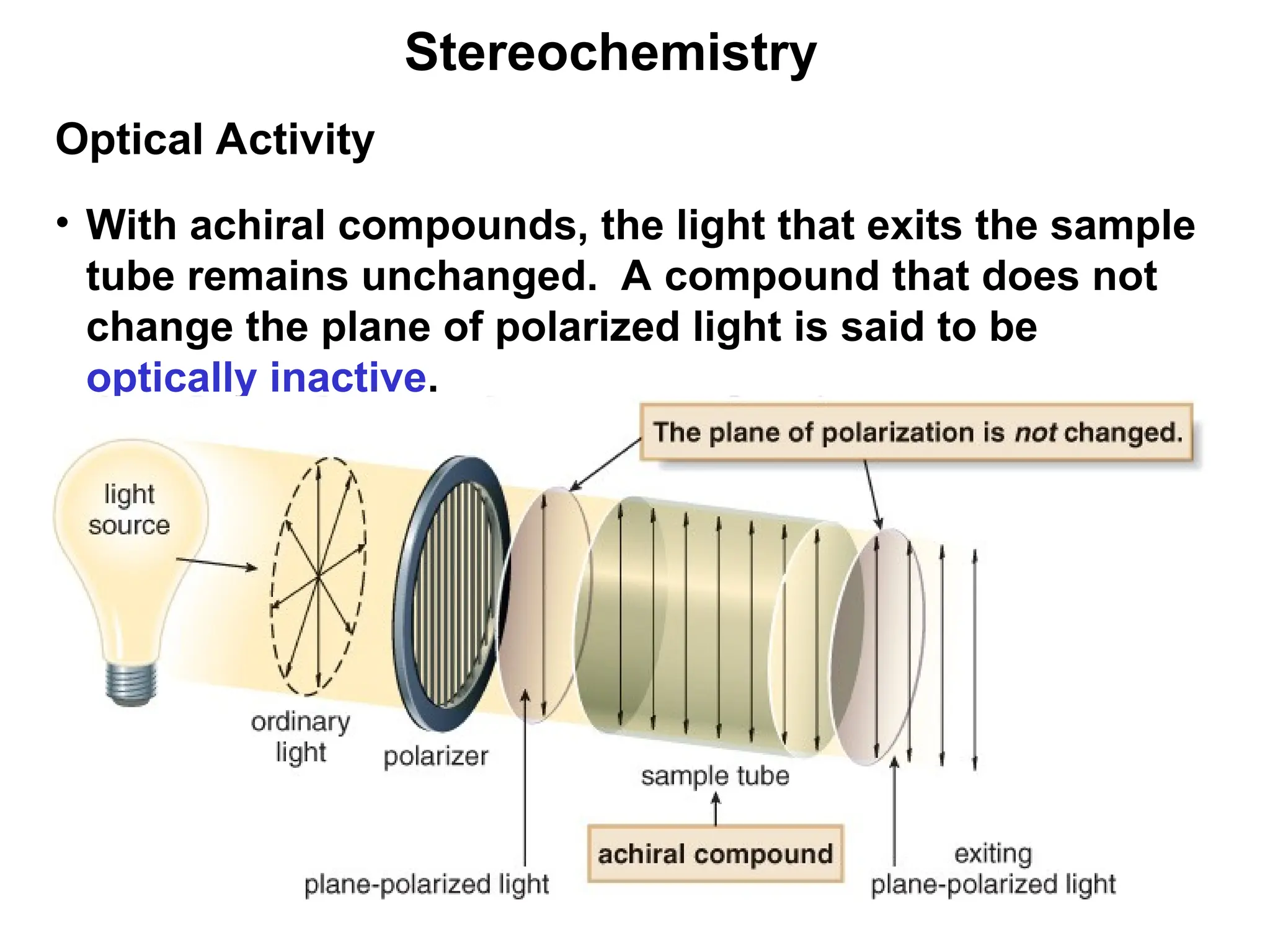
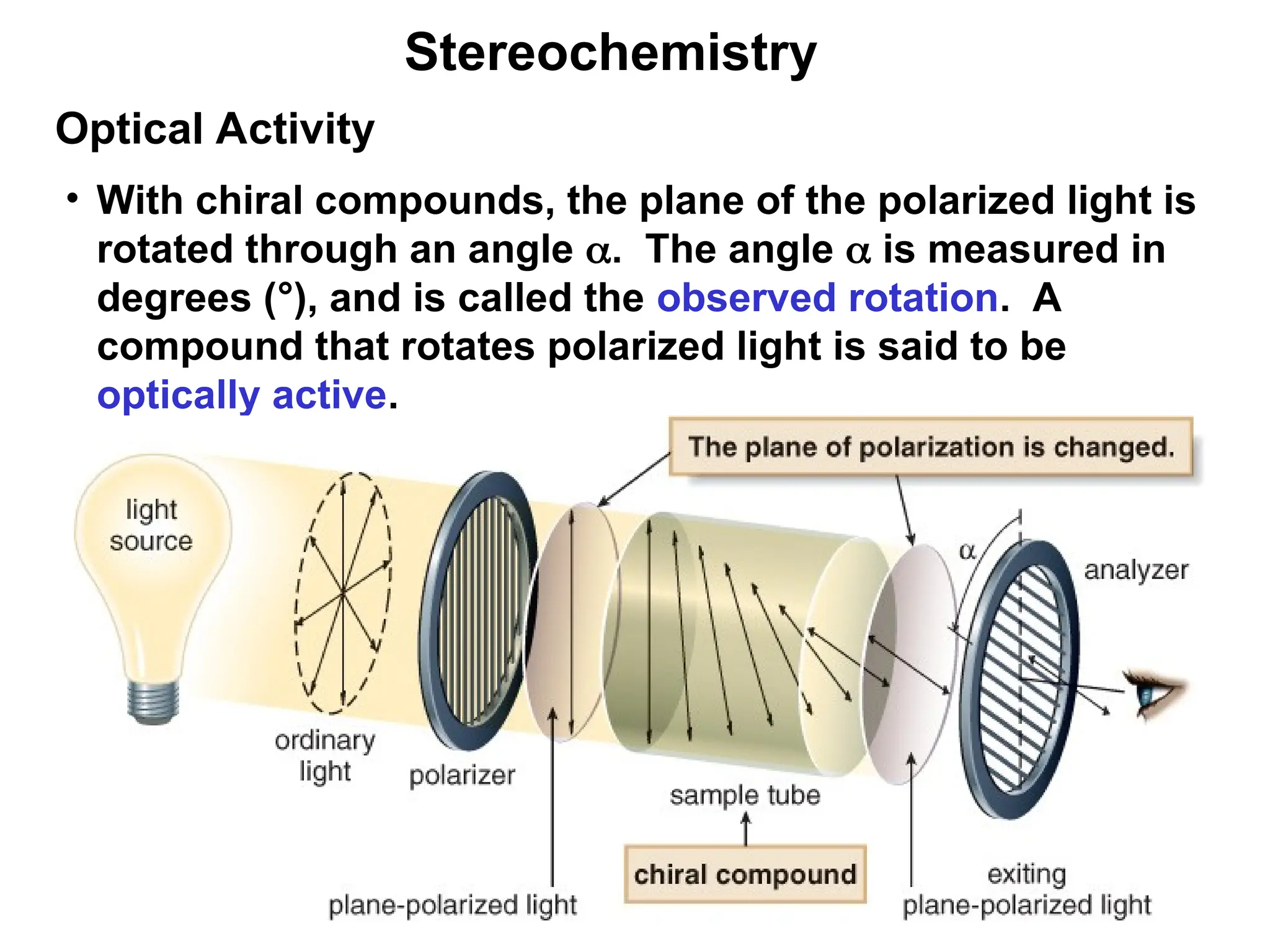
The document outlines the classification of isomers into constitutional isomers and stereoisomers, detailing their differences in structure and properties. It explains chirality, stating that a molecule is chiral if its mirror image is not superimposable, and describes the R/S nomenclature system for naming enantiomers. Furthermore, it discusses the concept of optical activity, including the behavior of chiral compounds with plane-polarized light, and introduces meso compounds and diastereomers.