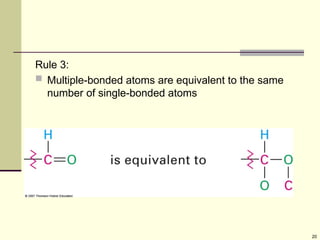
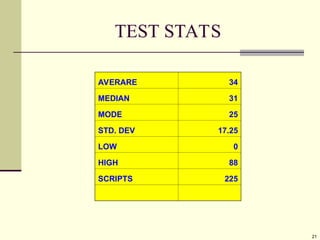
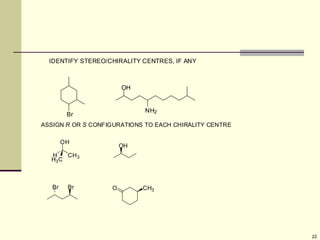
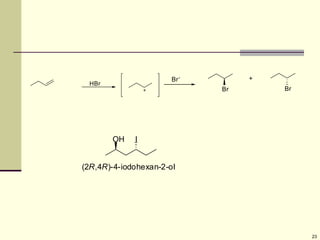
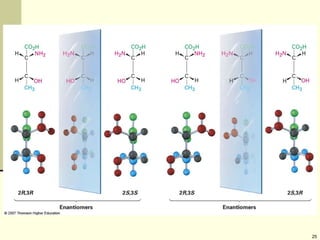
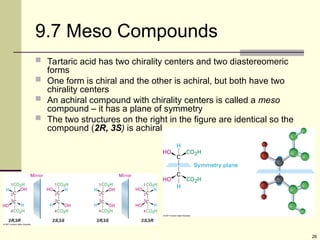
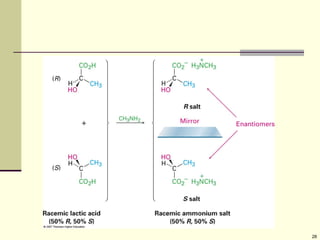
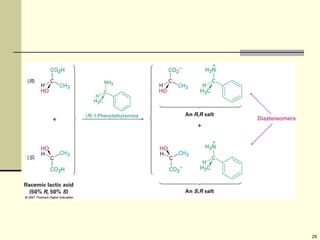
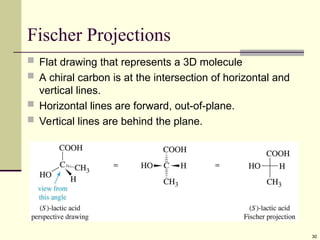
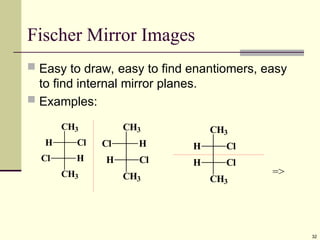
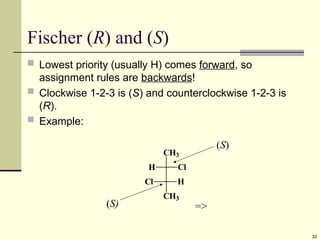
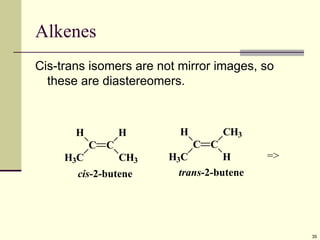
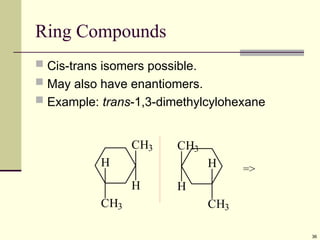
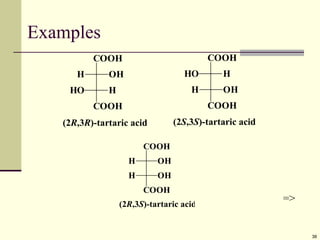
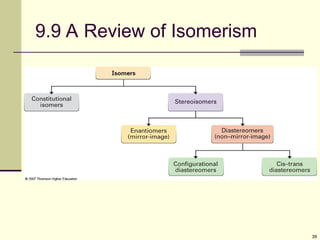
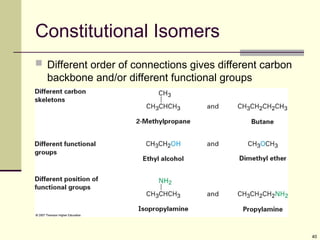
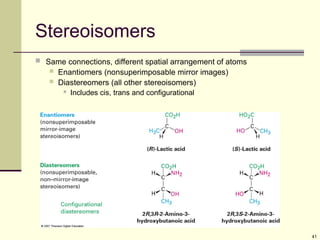
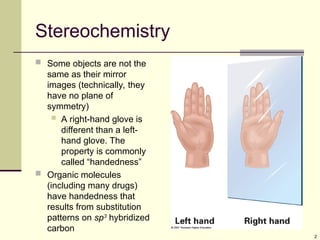
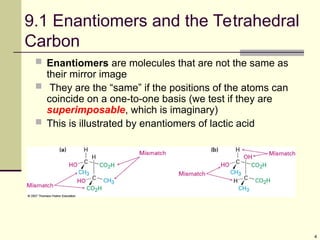
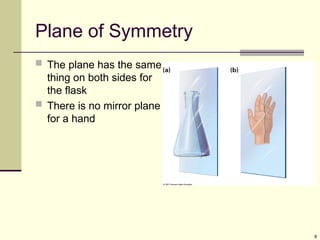
Explain in details the Some objects are not the same as their mirror images (technically, they have no plane of symmetry)
A right-hand glove is different than a left-hand glove. The property is commonly called “handedness”
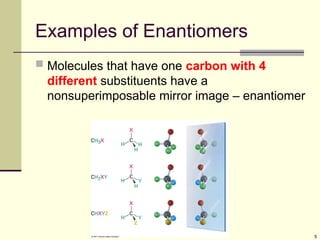
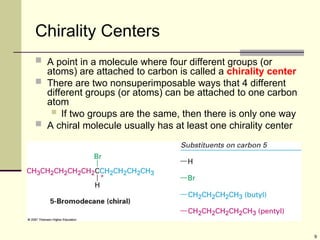
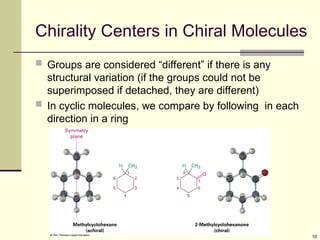
Organic molecules (including many drugs) have handedness that results from substitution patterns on sp3 hybridized carbon





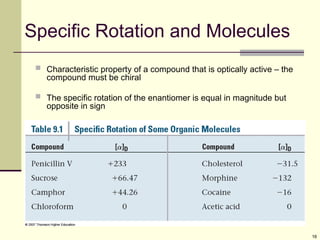
![12
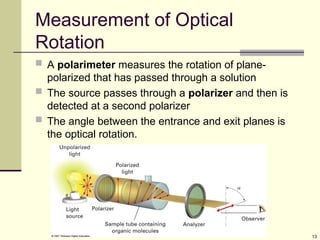
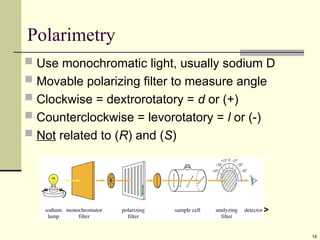
Optical Activity
Light passes through a plane polarizer
Plane polarized light is rotated in solutions of optically
active compounds
Measured with polarimeter
Rotation, in degrees, is []
Clockwise rotation is called dextrorotatory
Anti-clockwise is levorotatory](https://image.slidesharecdn.com/stereochemistry1-250610134630-9224ea2c/85/STEREOCHEMISTRY-1-for-year-2-stdents-ppt-12-320.jpg)
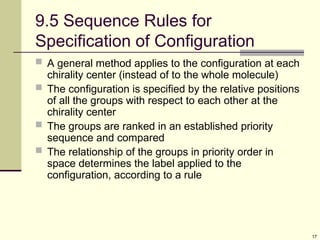
![15
Specific Rotation
To have a basis for comparison, define specific
rotation, []D for an optically active compound
[]D = observed rotation/(pathlength x concentration)
= /(l x C) = degrees/(dm x g/mL)
Specific rotation is that observed for 1 g/mL in
solution in cell with a 10 cm path using light from
sodium metal vapor (589 nm)](https://image.slidesharecdn.com/stereochemistry1-250610134630-9224ea2c/85/STEREOCHEMISTRY-1-for-year-2-stdents-ppt-15-320.jpg)