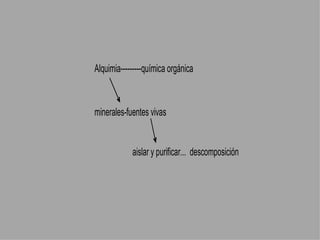
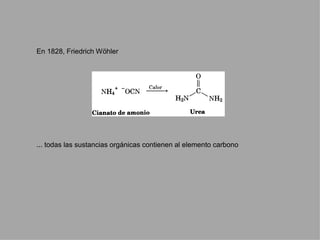
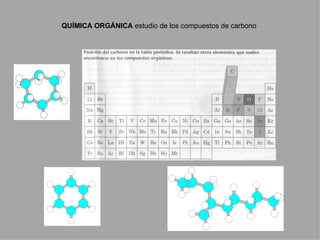
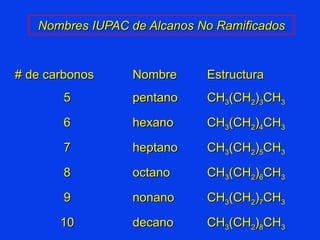
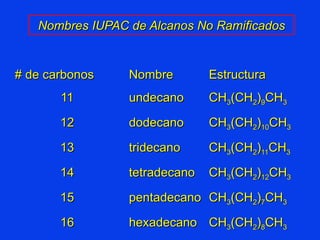
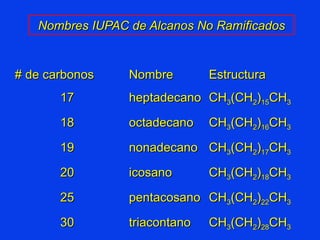
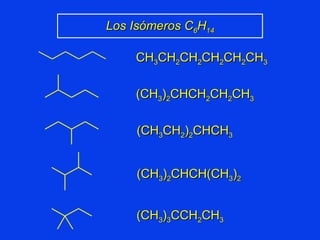
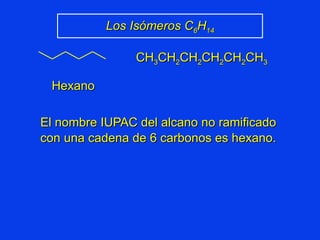
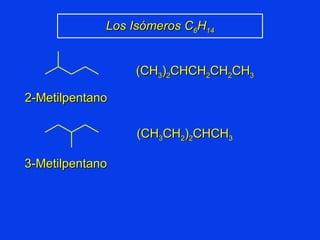
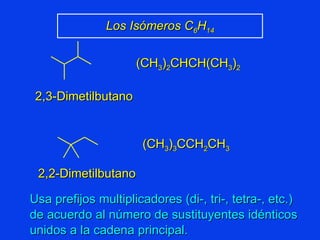
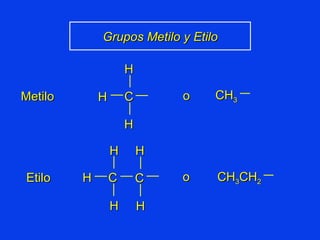
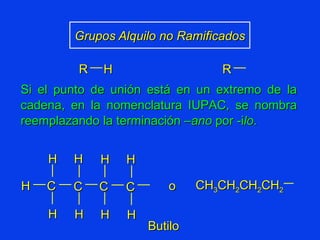
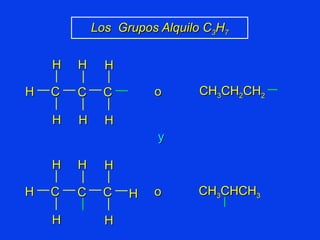
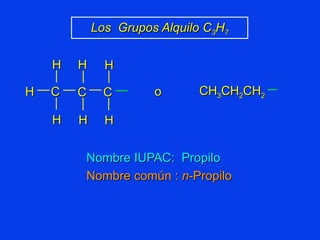
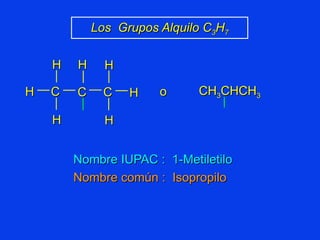
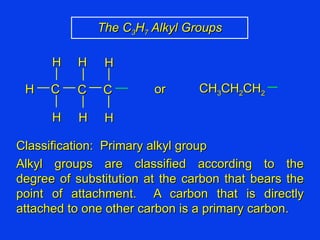
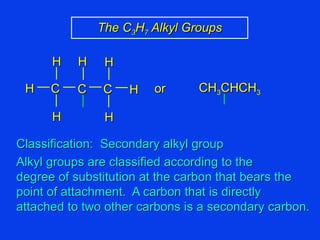
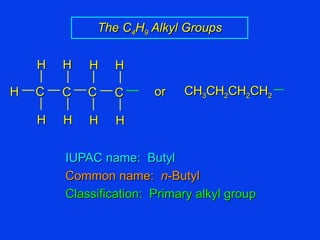
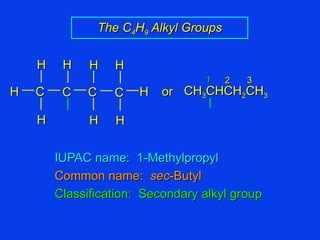
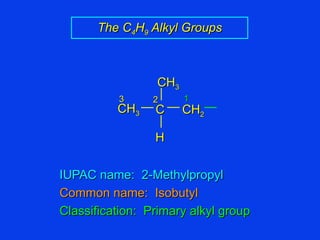
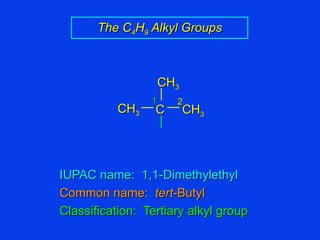
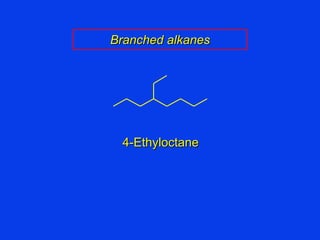
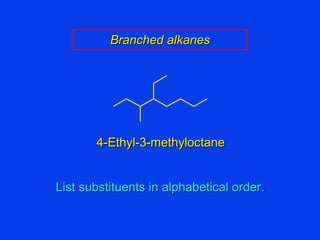
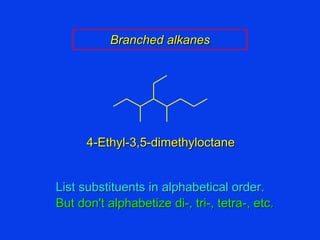
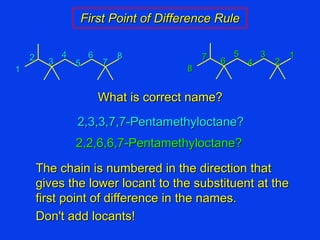
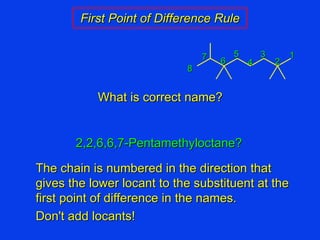
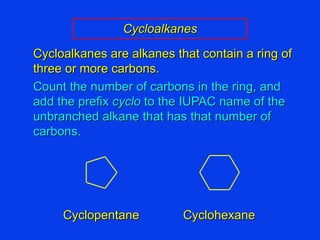
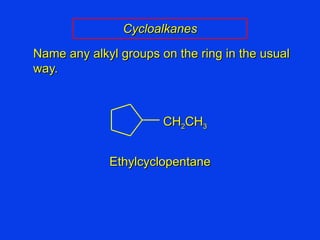
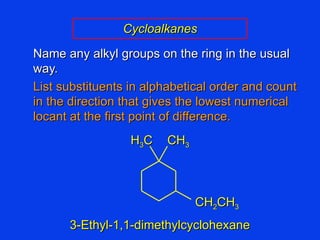
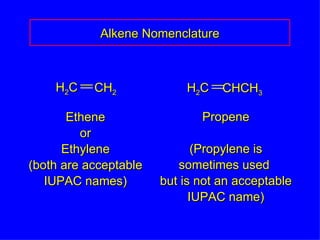
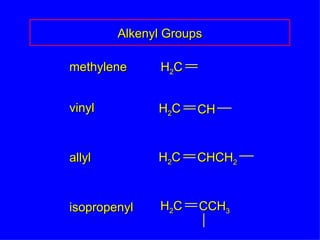
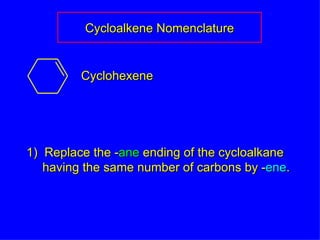
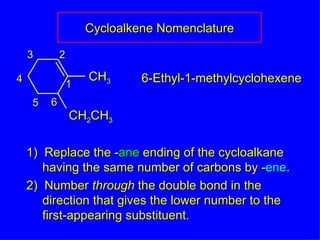
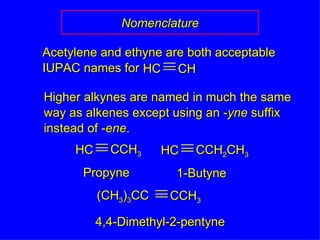
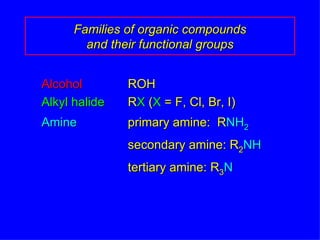
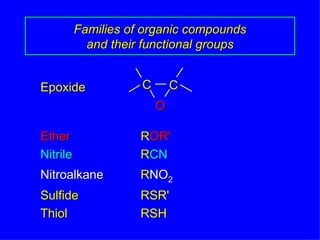
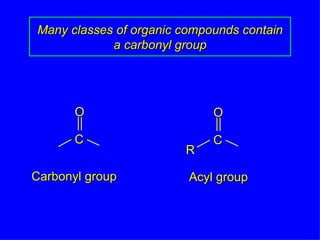
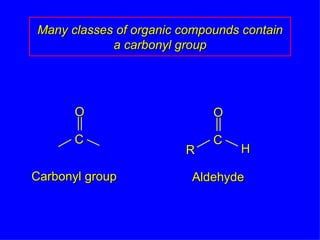
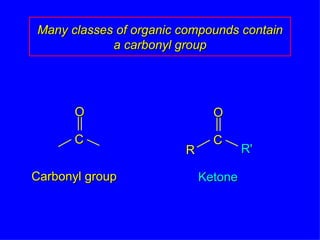
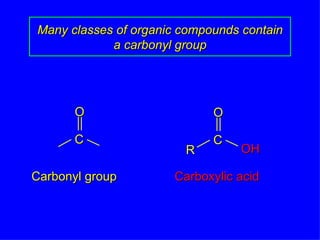
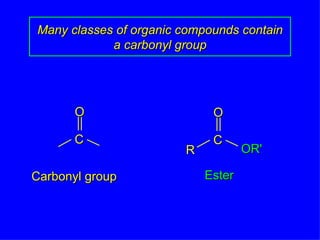
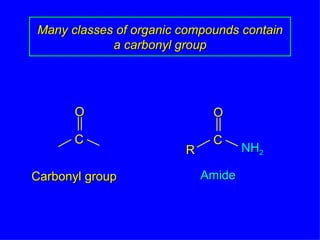
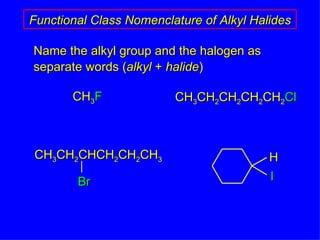
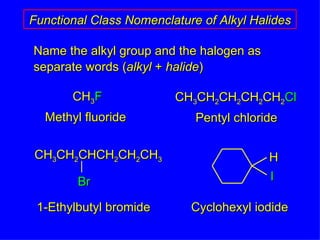
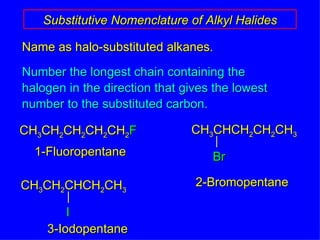
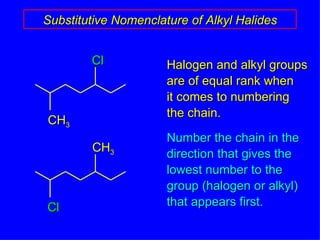
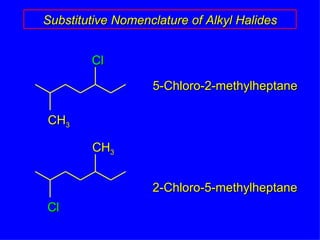
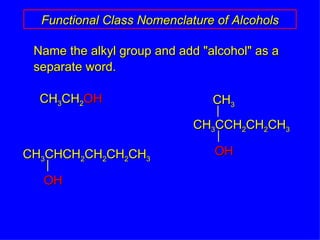
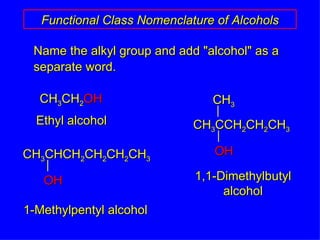
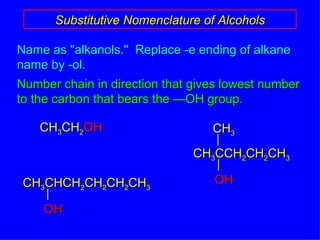
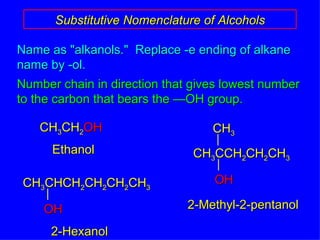
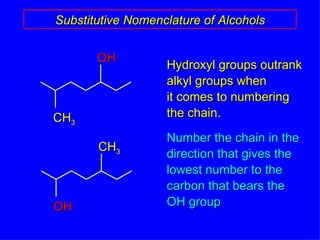
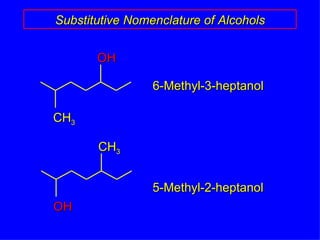
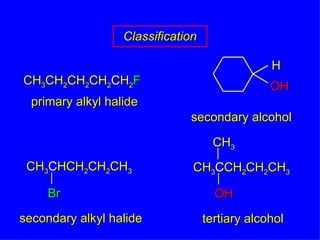
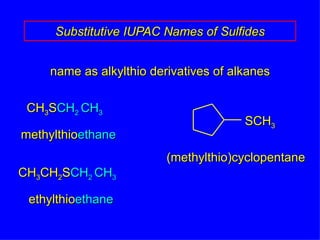
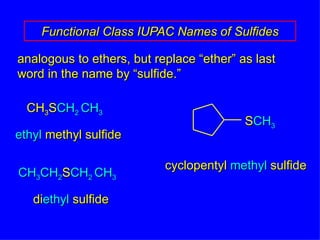
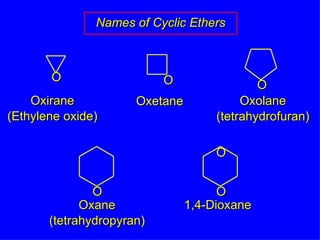
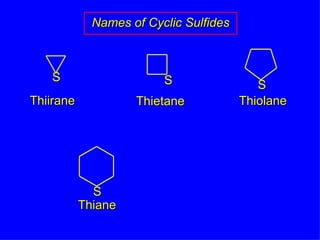
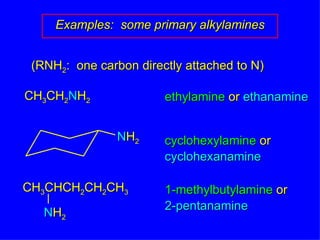
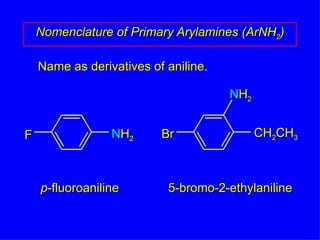
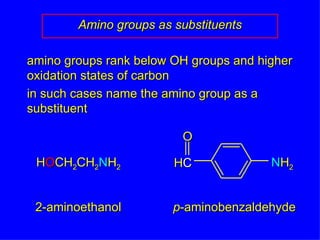
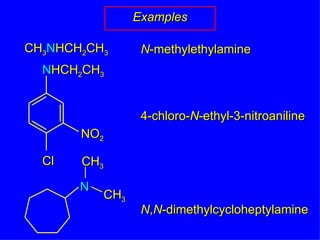
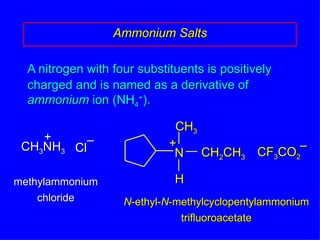
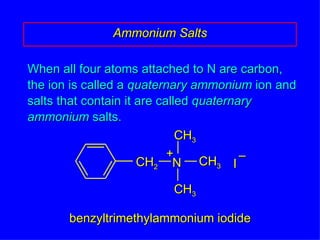
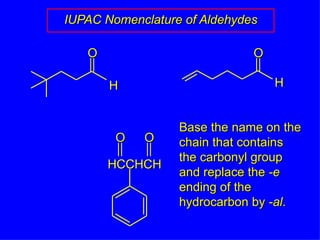
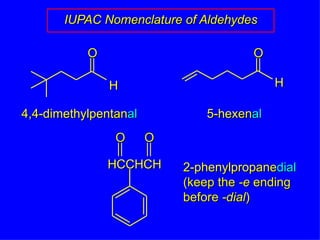
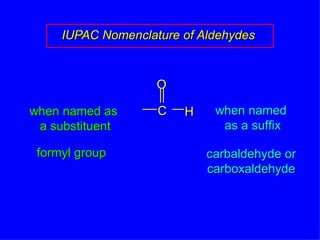
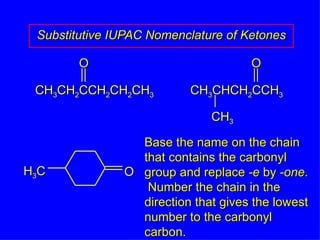
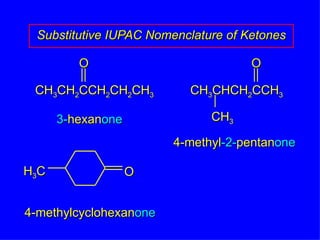
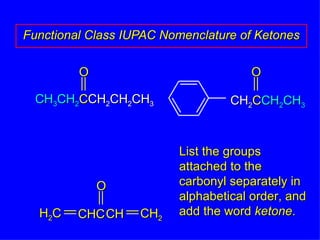
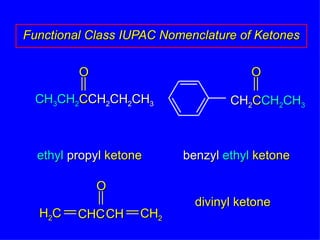
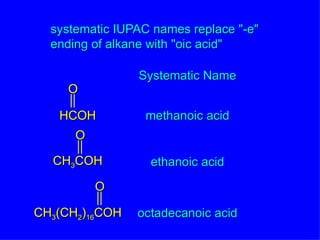
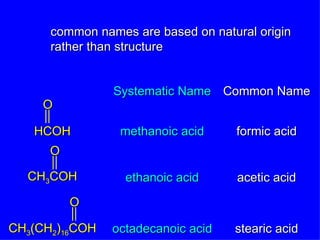
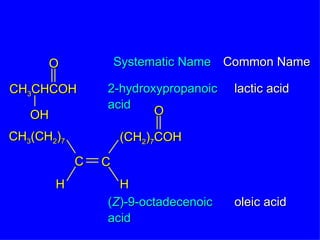
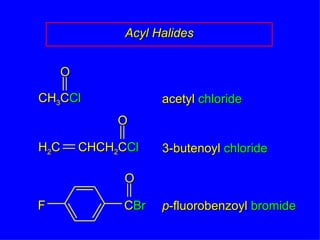
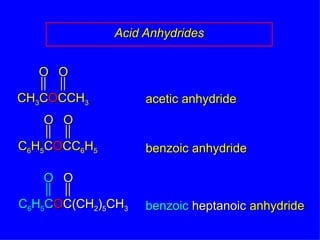
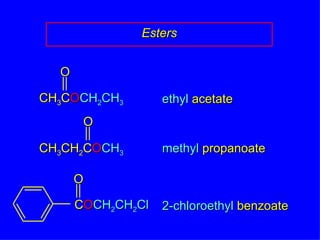
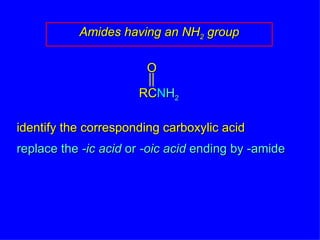
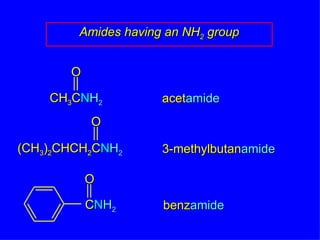
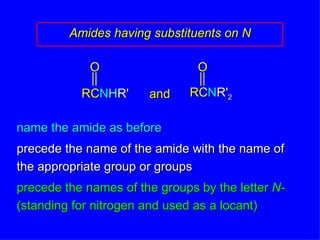
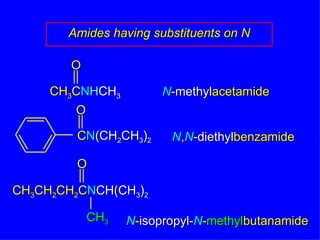
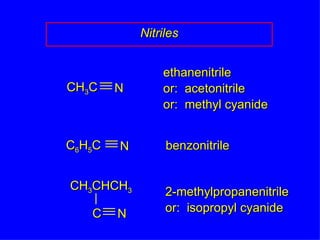
Organic chemistry is the study of carbon compounds found in living things like proteins, DNA, foods, medicines, and more. It describes the structures and properties of these compounds. The document provides examples of IUPAC naming conventions for alkanes, alkenes, alkynes, cycloalkanes, alcohols, and alkyl halides including how to identify functional groups and classify substituents. Proper IUPAC nomenclature follows systematic rules to unambiguously name organic molecules.