Embed presentation
Download to read offline
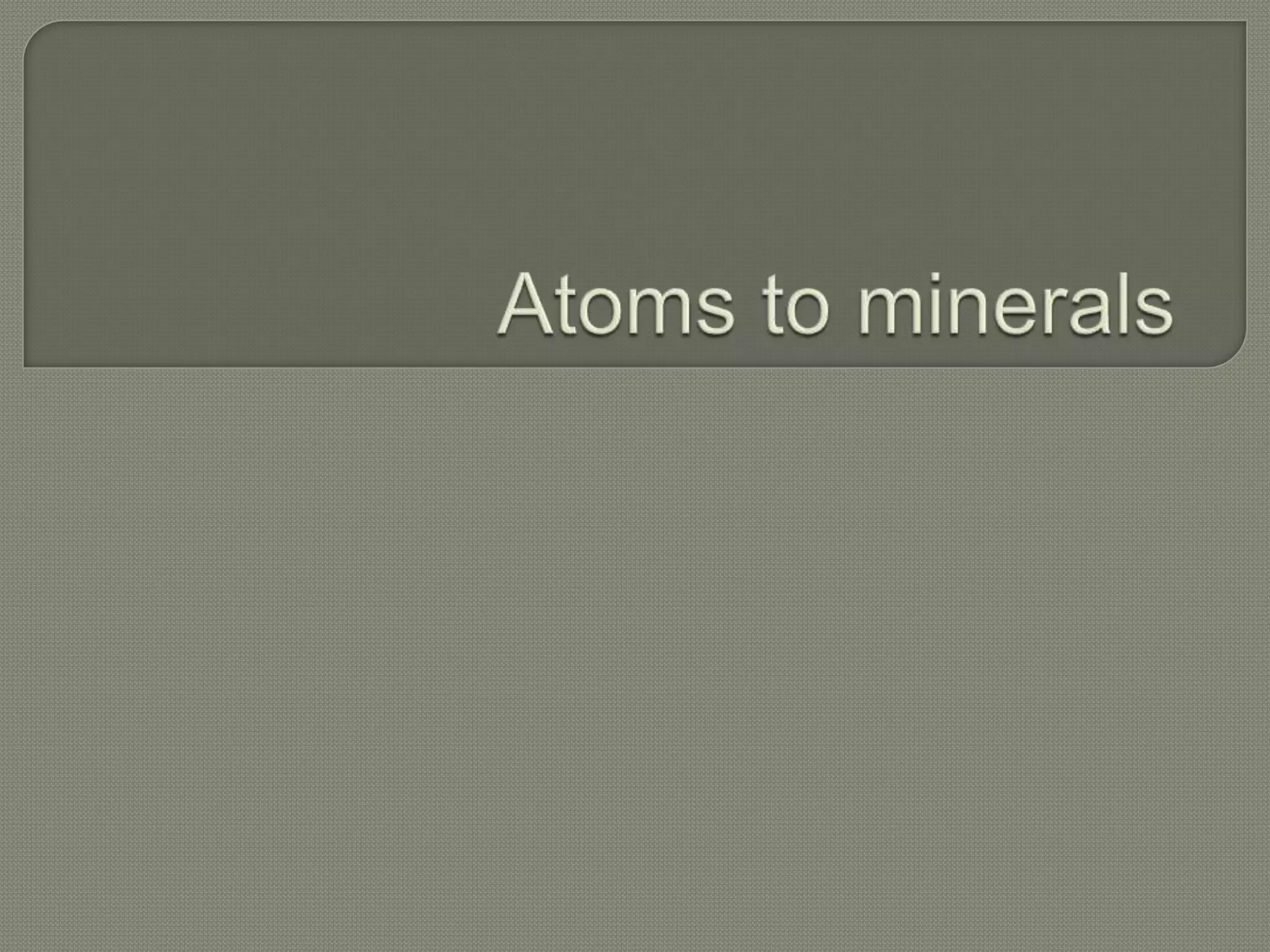

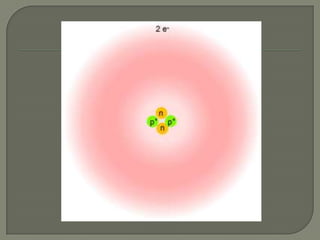
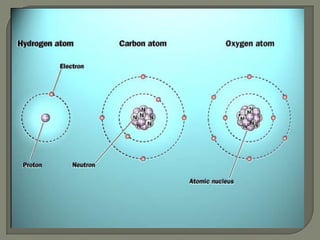
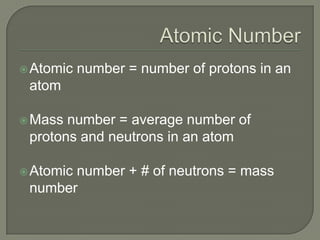


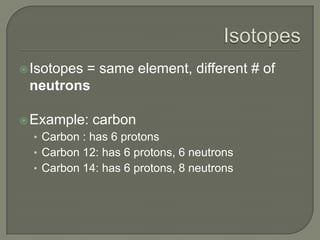
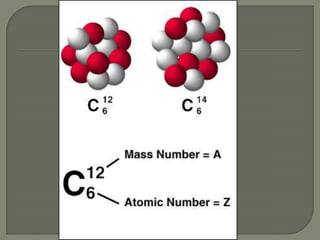







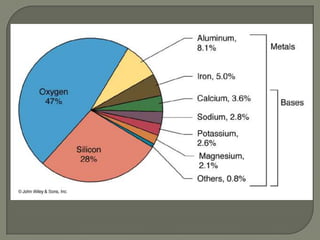
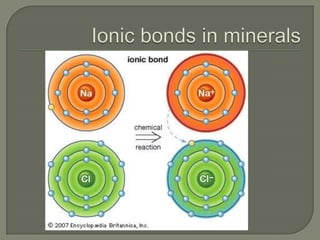
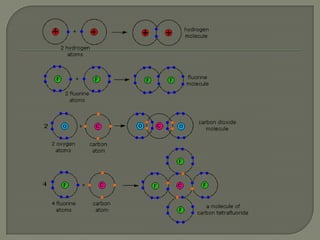
Atoms are the smallest units that make up elements and are composed of protons, neutrons, and electrons. Atoms combine to form compounds with different properties than the individual elements. Minerals are naturally occurring inorganic solids with a defined crystalline structure and chemical composition. Common minerals include quartz, halite, and diamonds, which are made up of elements like silicon, sodium, chlorine, and carbon bonded together in specific ratios.


















