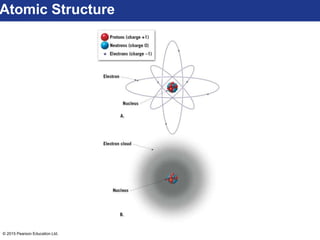
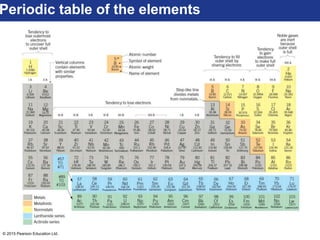
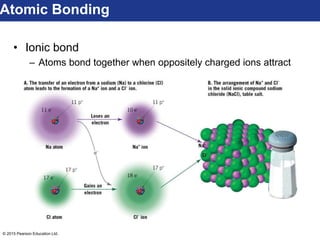
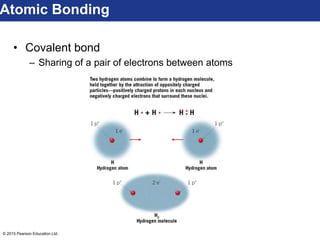
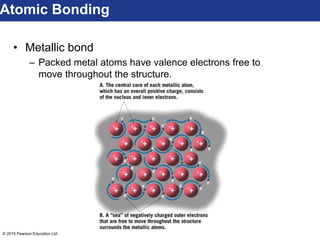
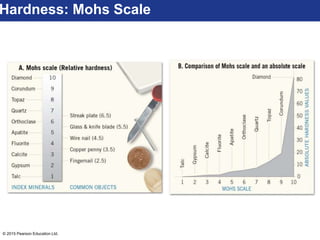
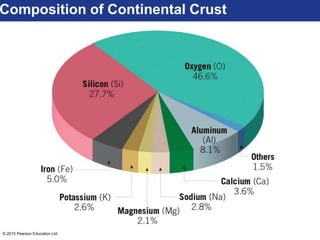
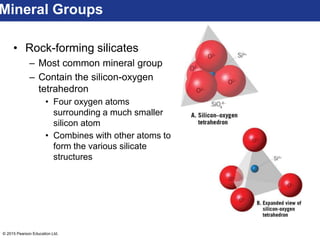
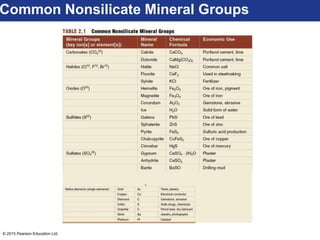
This document discusses minerals and their properties. It defines minerals as natural, inorganic solids with an internal atomic structure and definite chemical composition. Minerals are composed of elements, whose atoms bond together through ionic, covalent, or metallic bonds. The document outlines several key mineral groups including silicates, oxides, sulfides, carbonates, and halides. It notes that silicates are the most abundant mineral group in the Earth's crust and describes their tetrahedral structures. The document also discusses physical properties of minerals like hardness, luster, and cleavage. Finally, it distinguishes between renewable and nonrenewable natural resources, with minerals being nonrenewable.