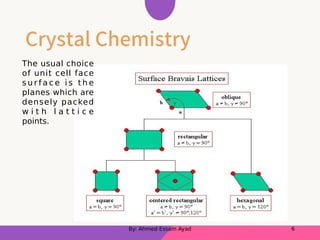
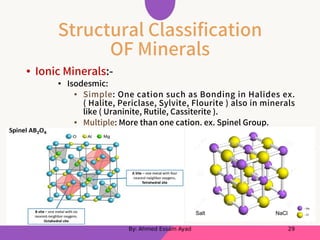
The document discusses structural mineralogy and crystal chemistry, explaining key concepts such as lattice structures, unit cells, and coordination numbers. It categorizes minerals based on their bonding types, including metallic, covalent, and ionic bonds, with examples of each. Additionally, it describes how ionic radius ratios influence the coordination of ions within mineral structures.