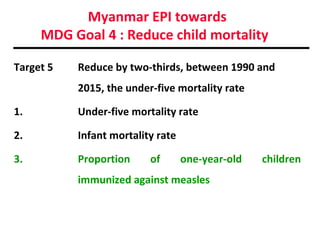
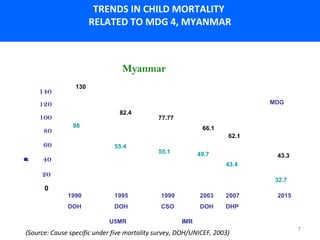
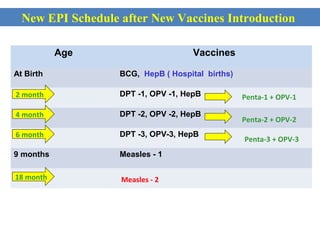
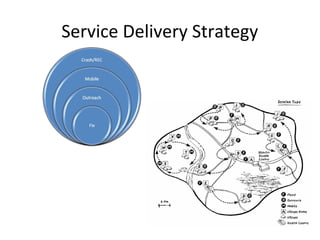
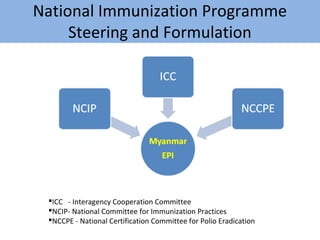
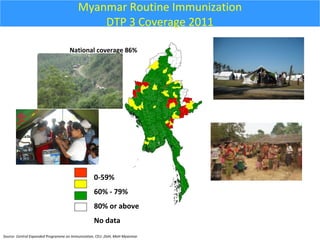
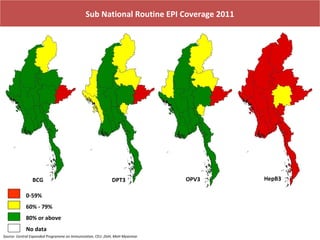
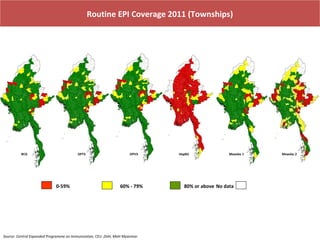
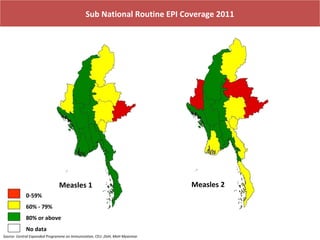
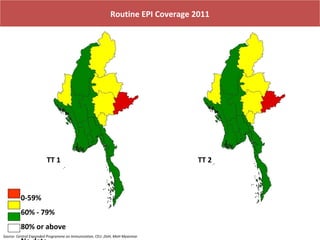
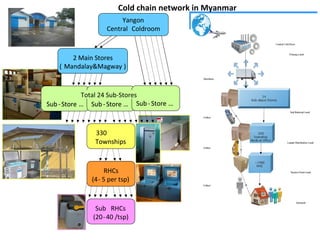
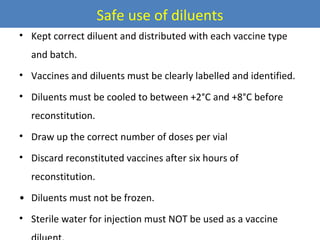
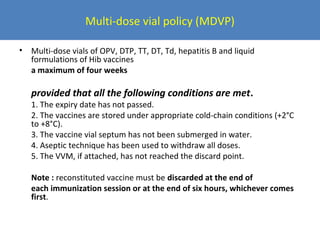
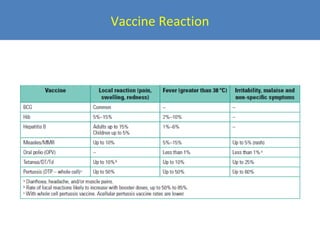
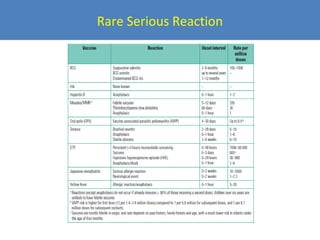
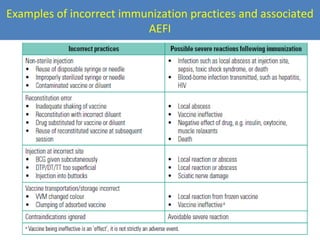
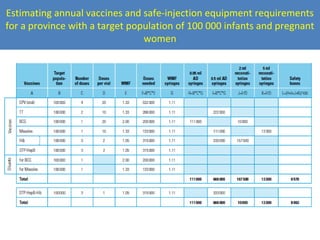
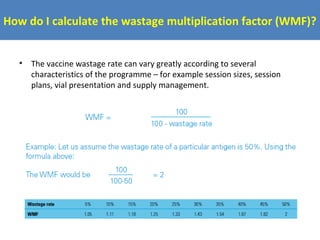
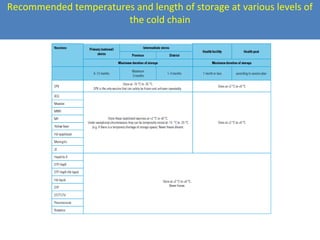
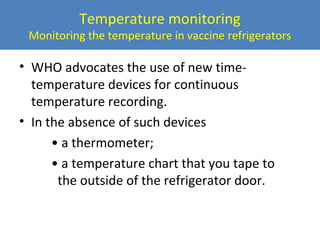
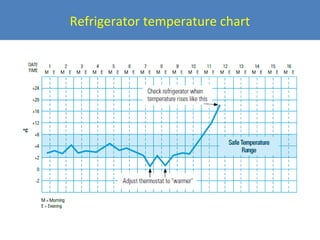
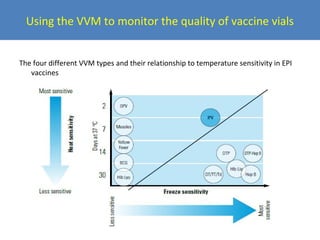
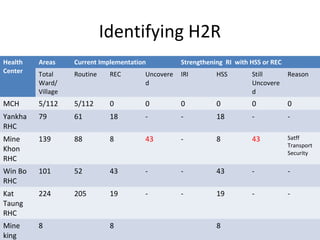
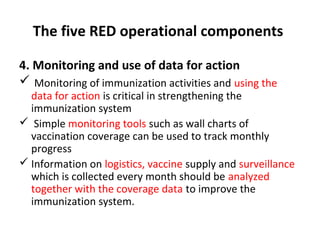
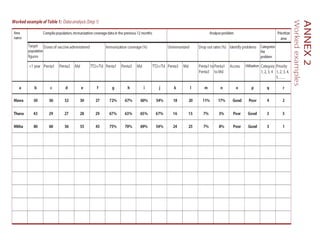
This document outlines the goals and objectives of Myanmar's National Immunization Program, including reducing under-5 mortality from vaccine-preventable diseases and reaching routine immunization coverage targets. It discusses strategies to strengthen routine immunization through the RED approach, which focuses on re-establishing outreach services, supportive supervision, community linkages, monitoring data use, and resource planning and management at the township level. Proper vaccine and equipment supply management, cold chain maintenance, and adverse event monitoring are also covered to help ensure vaccine quality and safety.