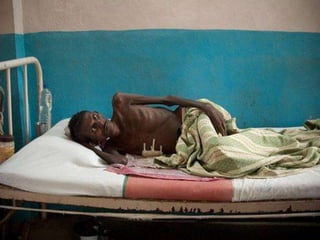
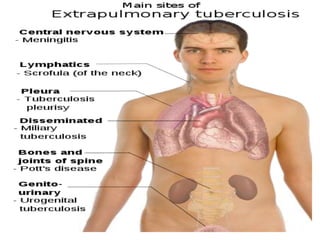
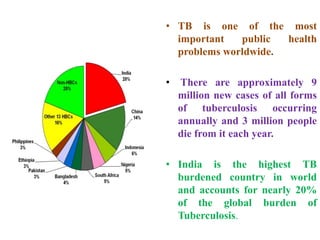
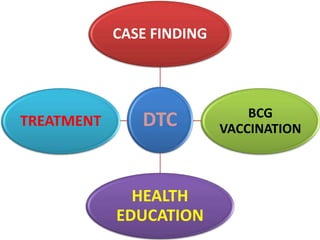
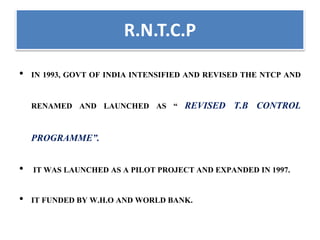
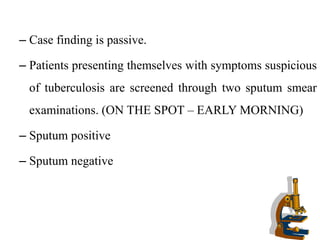
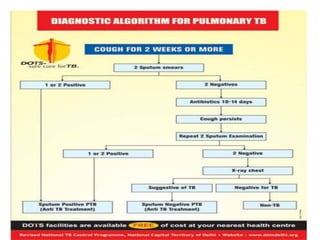
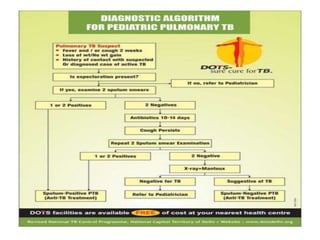
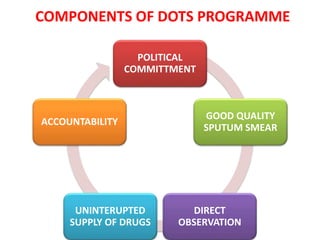
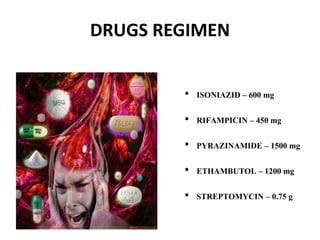
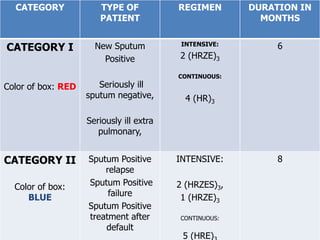
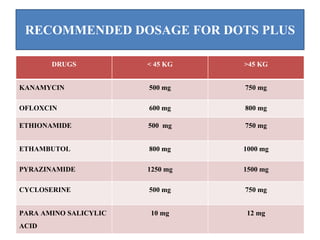
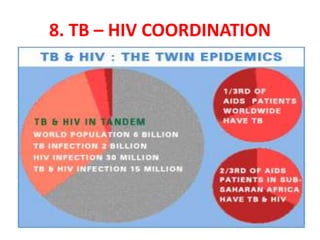
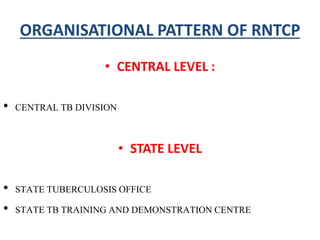
The document discusses India's National Tuberculosis Control Programme and its revisions. It notes that TB is a major public health problem worldwide and in India. The original programme had low case detection and treatment results. An expert committee recommended revisions in 1992, leading to the launch of the Revised National Tuberculosis Control Programme in 1993 with DOTS strategy, improved laboratories, and involvement of NGOs and private institutions. The revised programme has expanded nationwide and achieved reductions in death rates.