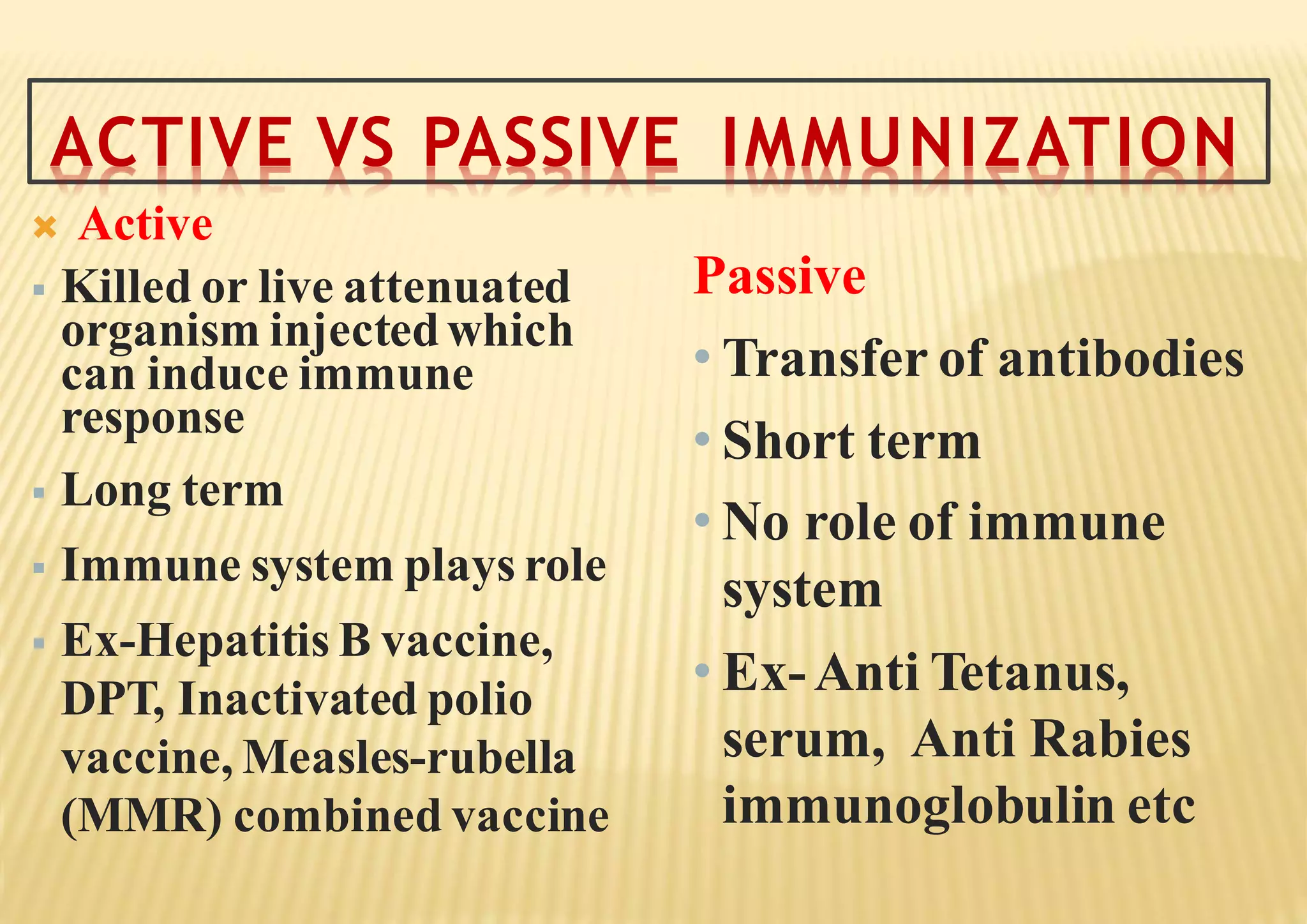
The document outlines the immunization schedule, detailing the history, importance, types of vaccines, and the universal immunization program. It emphasizes the significance of vaccinations in preventing diseases, and describes vaccine types and administration protocols, alongside the functioning of the cold chain necessary for vaccine efficacy. Additionally, it discusses strategies and objectives for the expanded immunization program aimed at achieving comprehensive coverage for children and women of childbearing age.