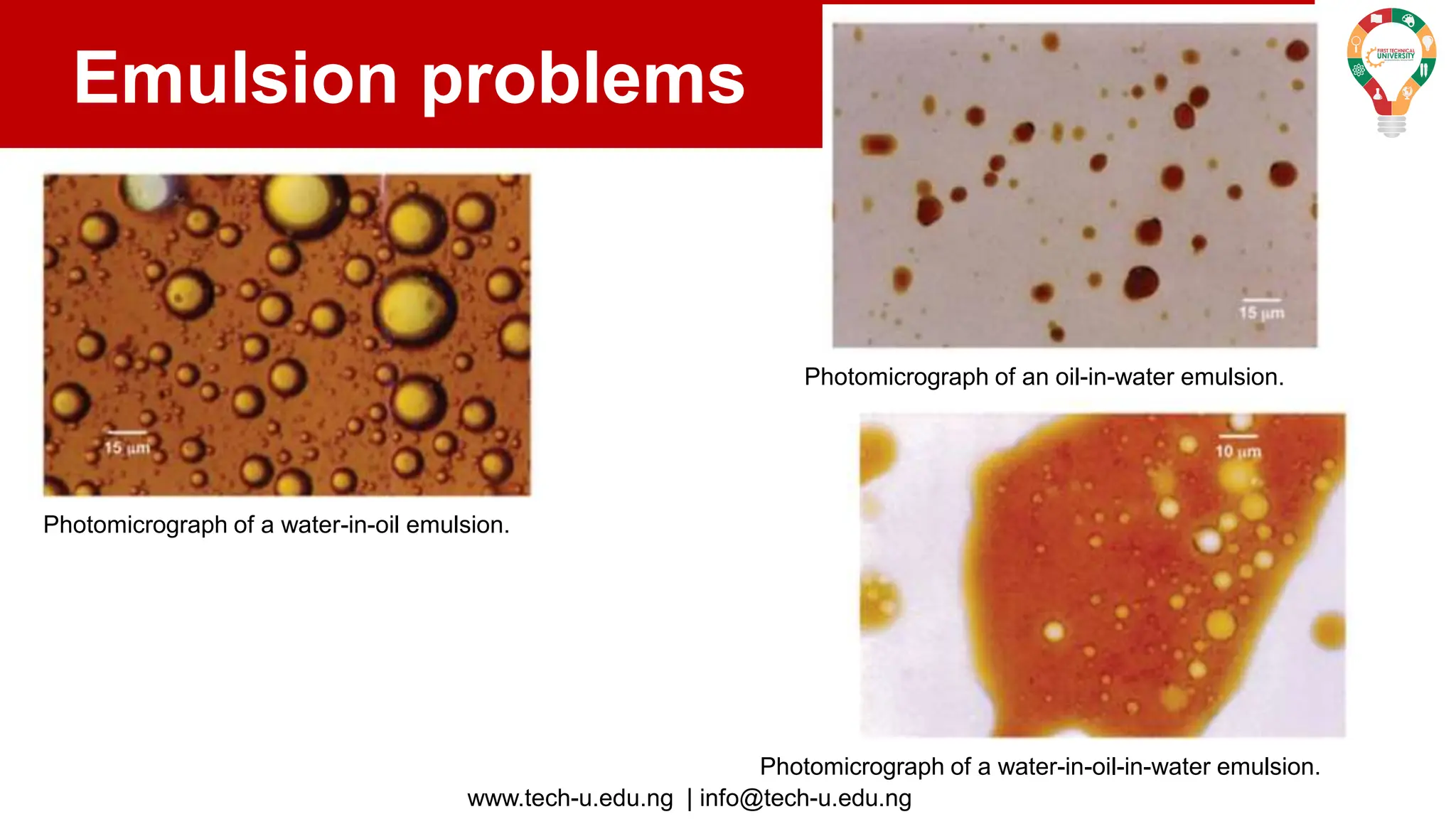
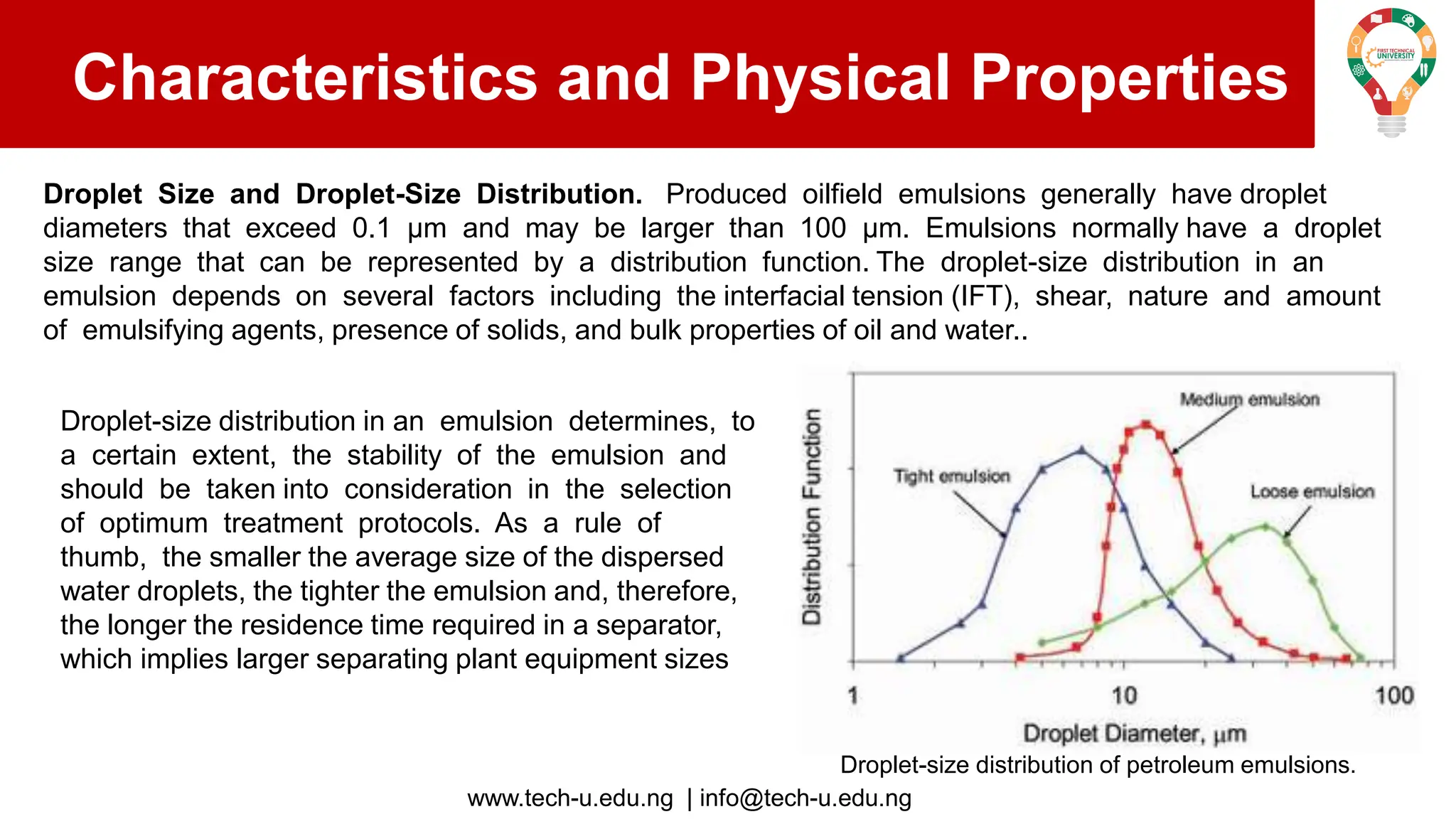
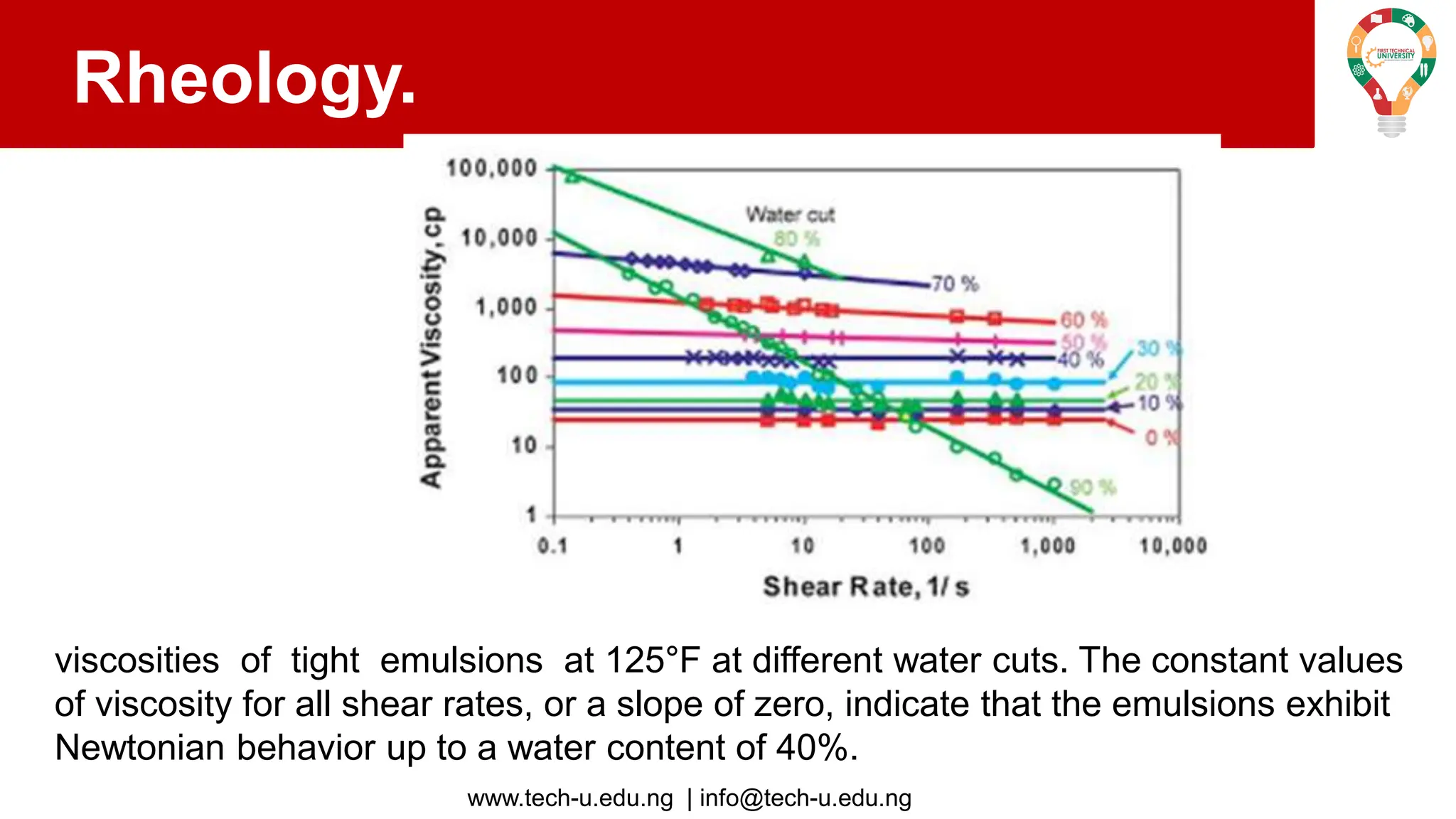
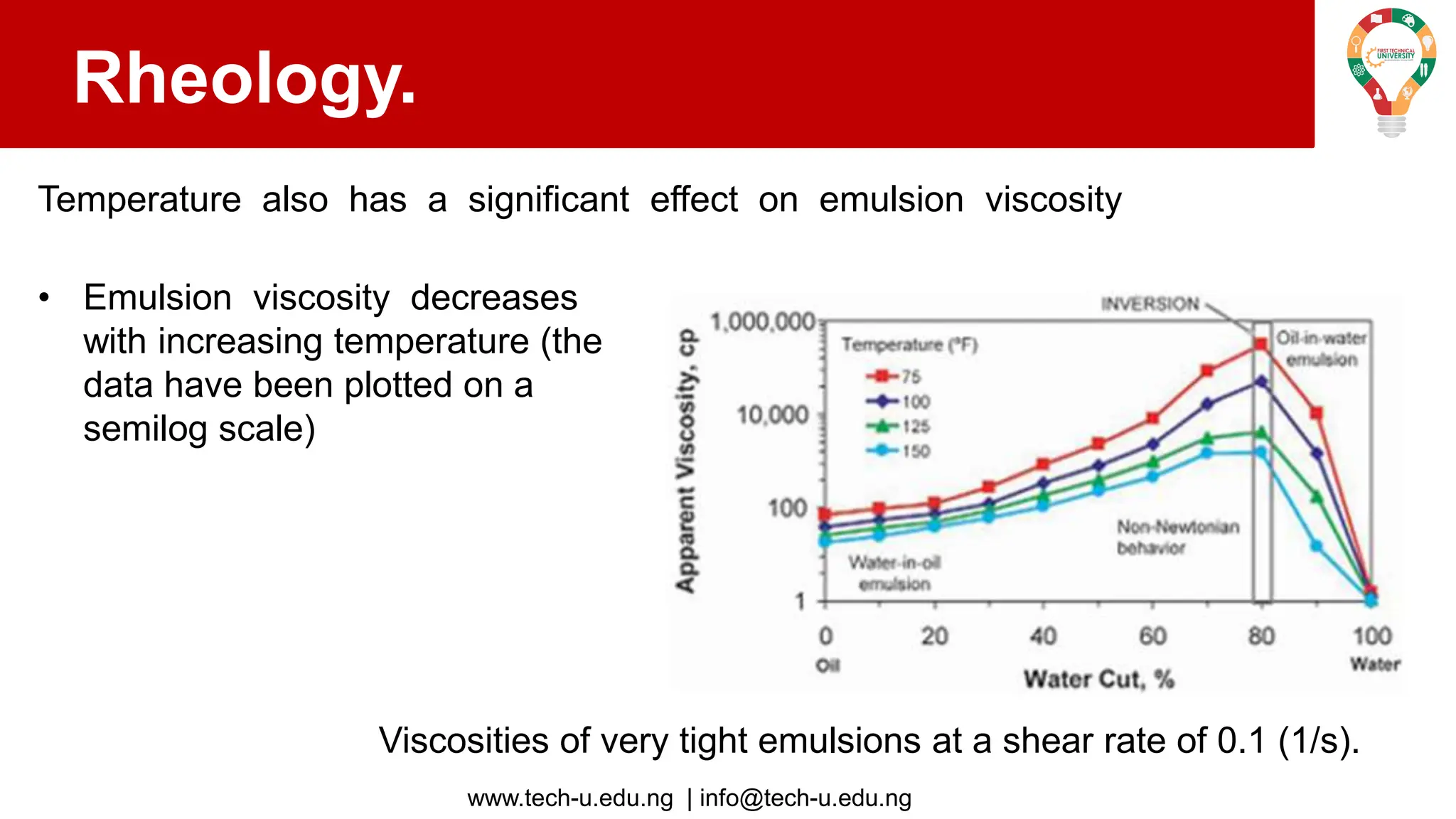
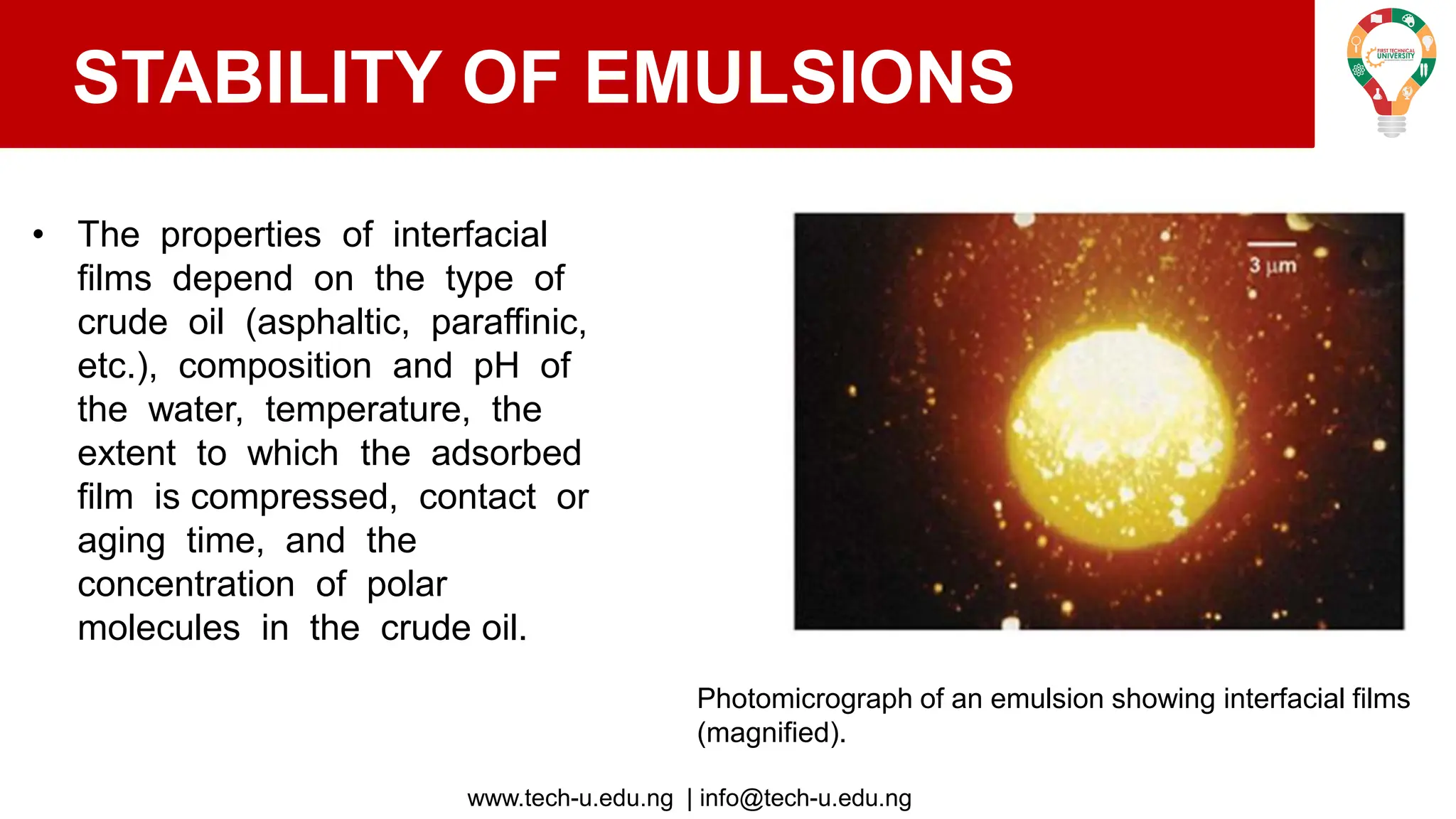
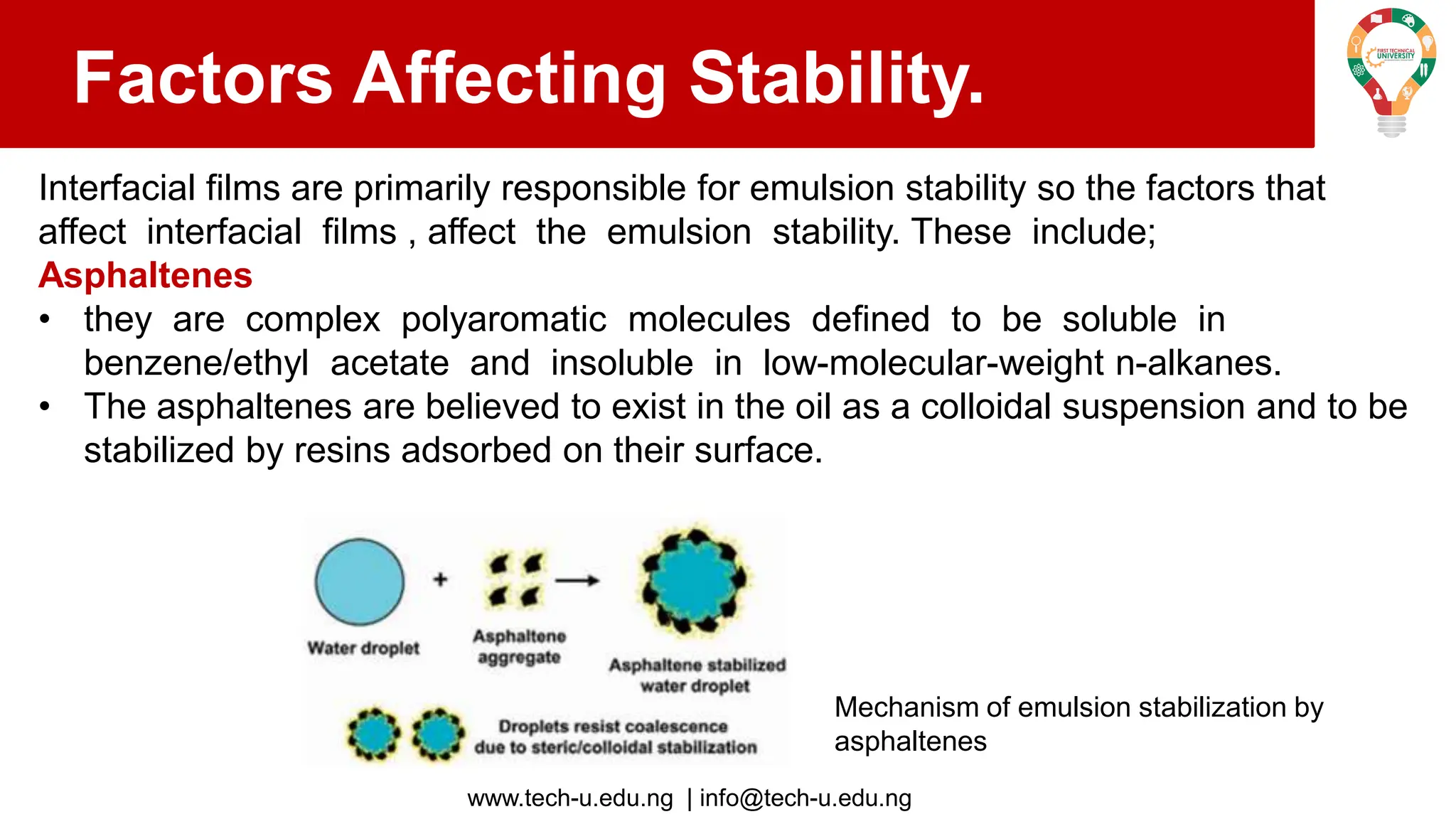
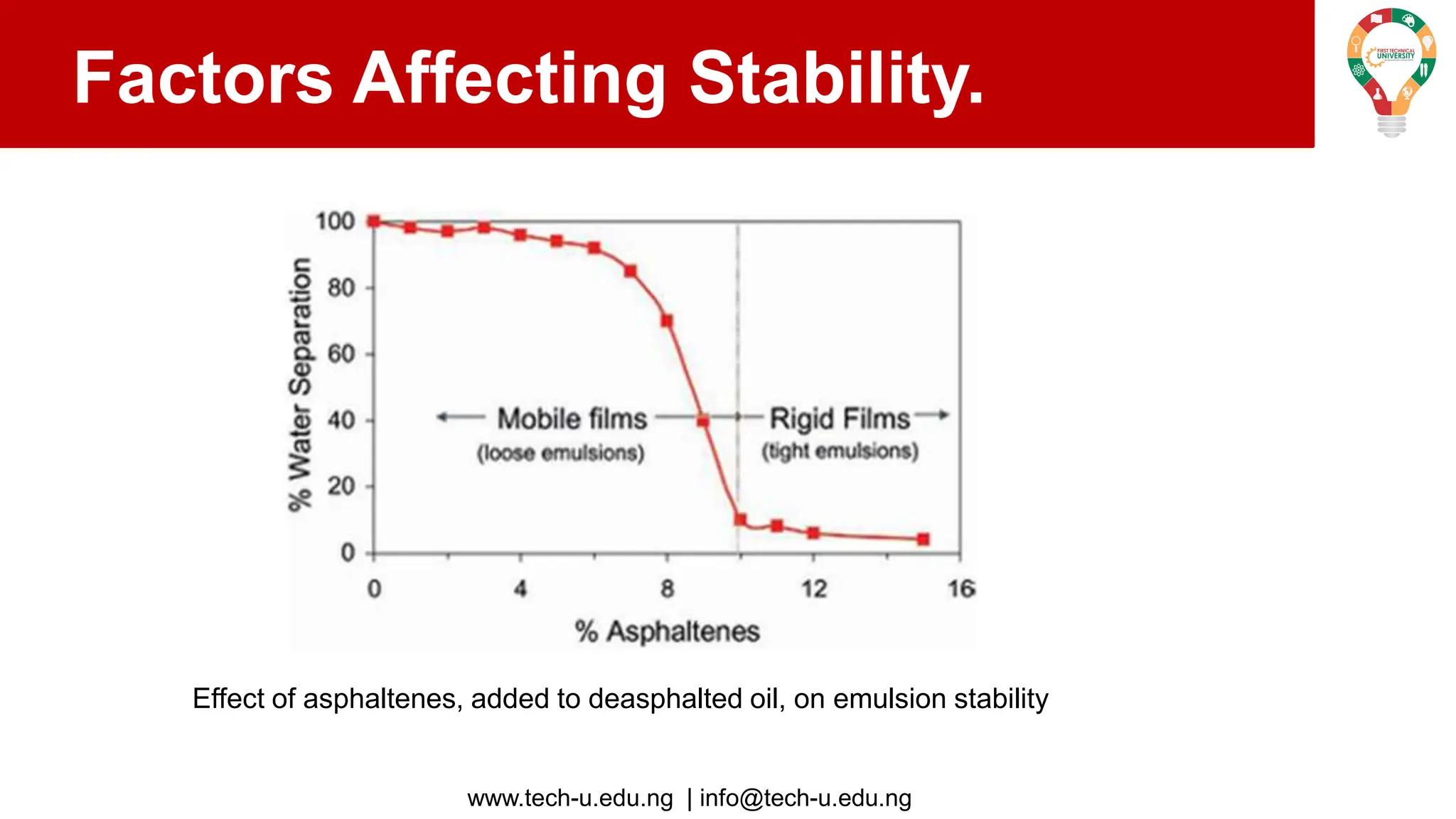
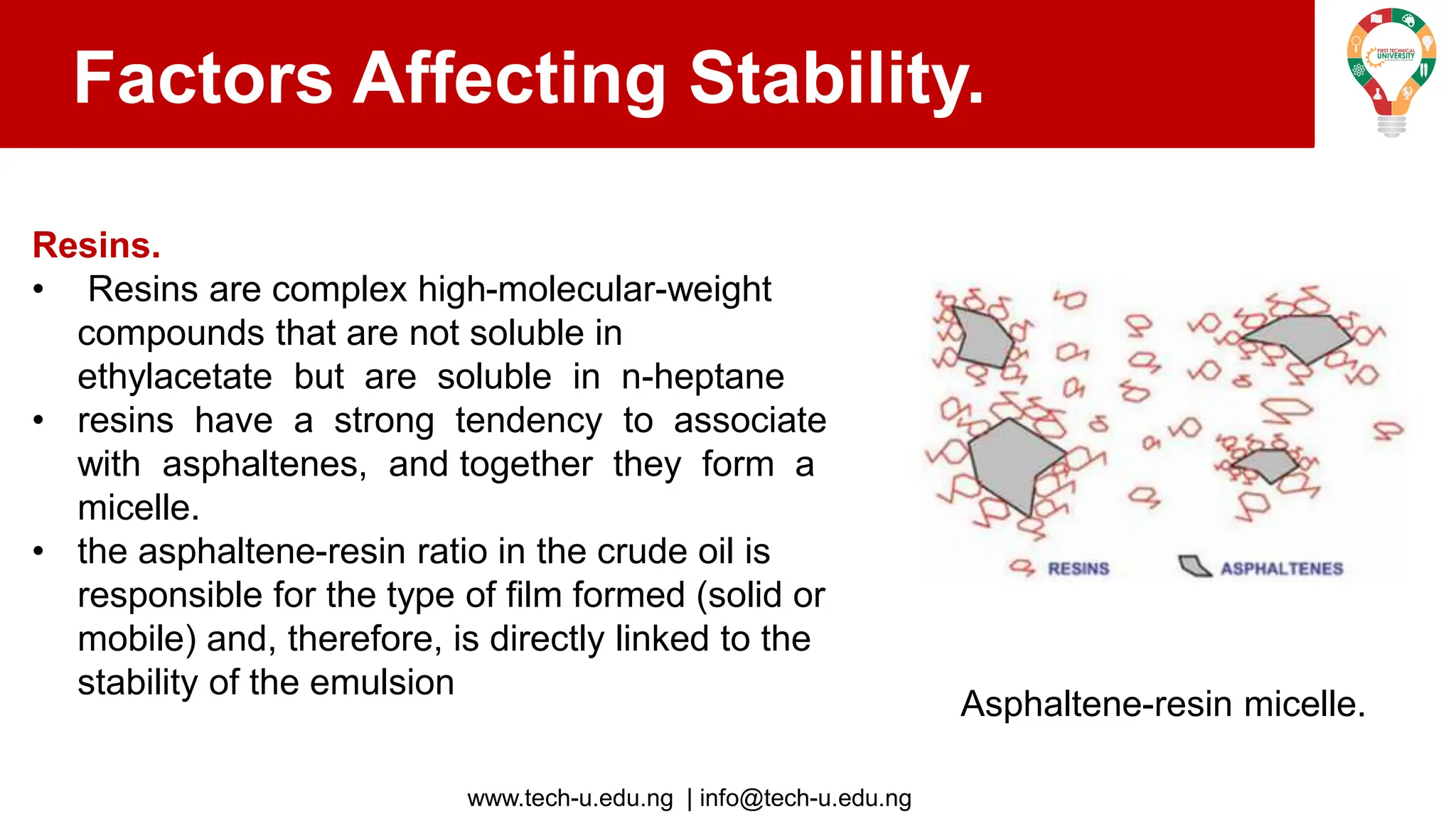
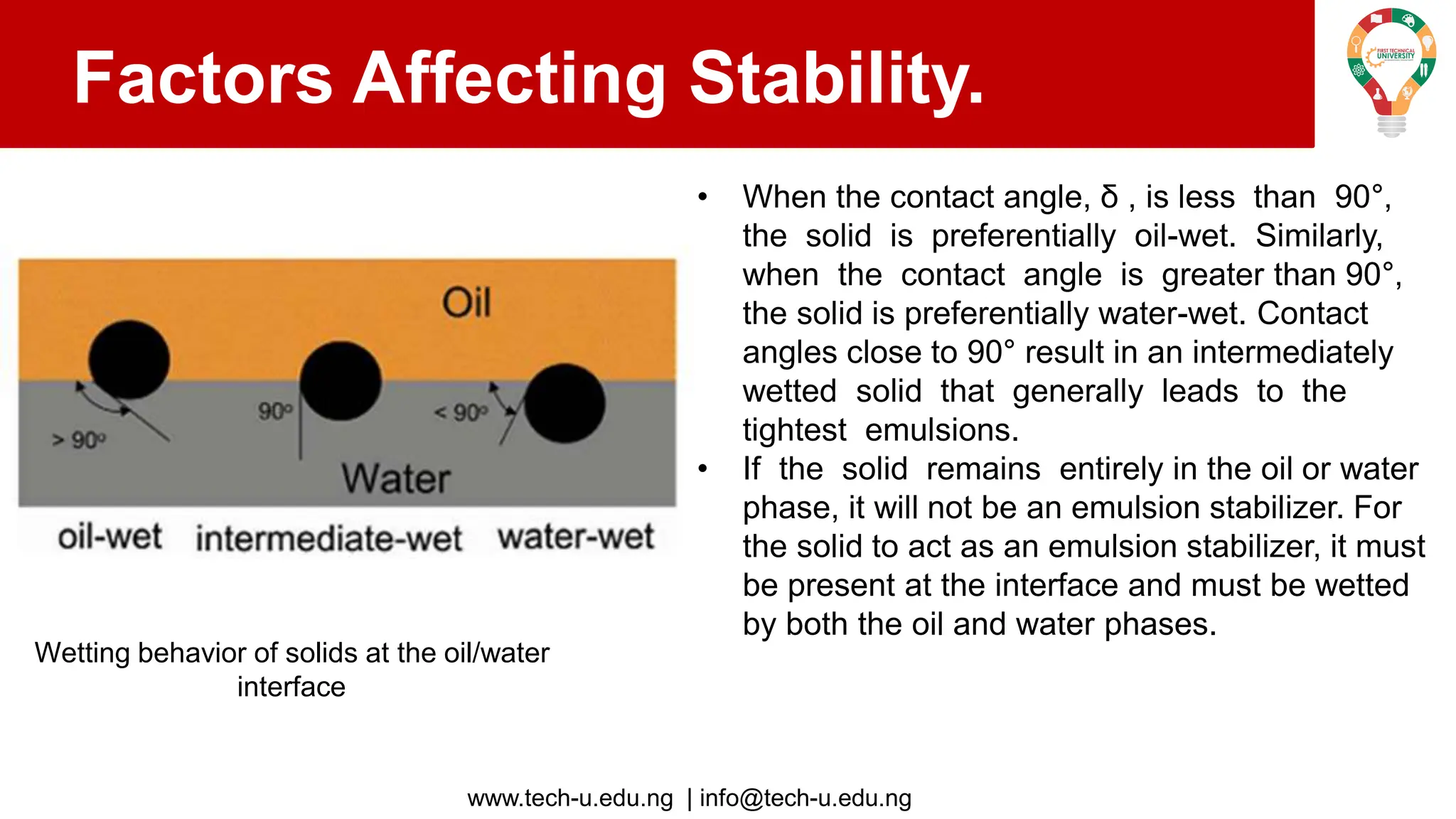
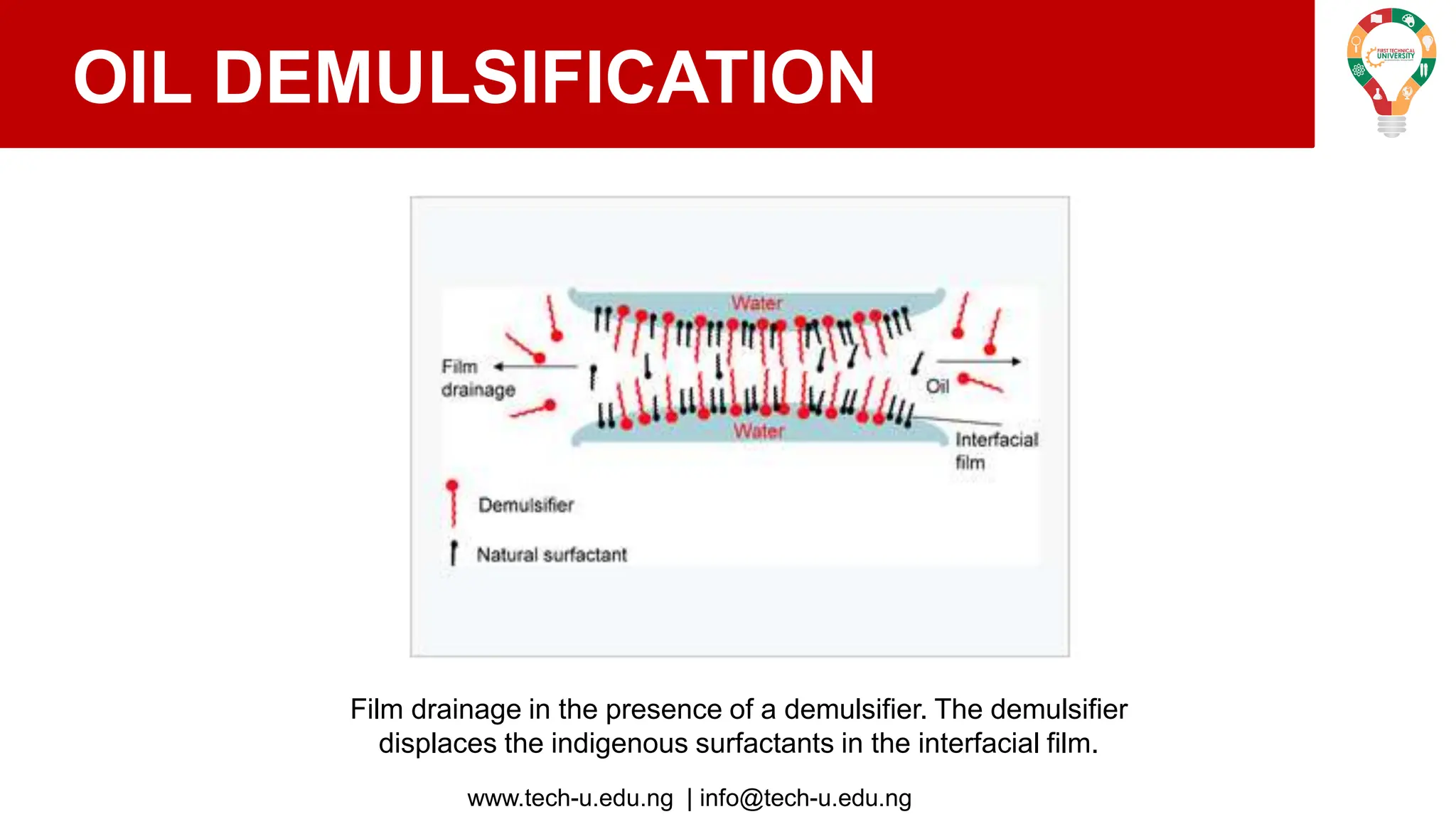
The document discusses various aspects of emulsions in petroleum production, including their definitions, types (such as water-in-oil and oil-in-water), formation processes, and the role of emulsifying agents. It details the physical characteristics and stability of emulsions, along with factors affecting their stability like temperature, droplet size, and pH. Additionally, it outlines demulsification processes to separate emulsions into oil and water phases, emphasizing methods to enhance separation efficiency.