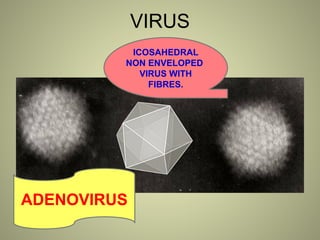
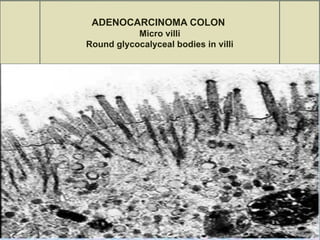
The document provides an extensive overview of electron microscopy (EM), including its history, types, components, and applications in diagnosing various diseases. It emphasizes the advantages of transmission electron microscopy (TEM) in achieving high resolution for studying subcellular structures, while detailing the intricate sample preparation processes required for effective imaging. Additionally, the document discusses specific applications of EM in renal and liver biopsies, highlighting its role in identifying metabolic and inherited diseases.















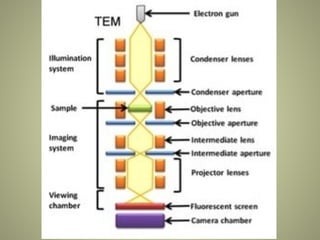









![SAMPLE
• The sample is placed in the microscope column below the
condenser with the help of a holder.
• Now the electrons interact with the thin tissue and hit the
atoms of the tissue.
• Heavier atoms deflect the electrons and are known as
“electron-dense” areas [ BLACK ].
• The electron passes through the lighter atoms and
produces an “electron-lucent / transparent” area
[ WHITE ].](https://image.slidesharecdn.com/electronmicroscope-241019151852-0f2713d1/85/ELECTRON-MICROSCOPE-pptx-26-320.jpg)










![COMBINED FIXATION TECHNIQUE
• At first the tissue is kept in 2% glutaraldehyde solution
for 2 h.
• After 2 h the fixative should be poured out, and the
tissue is washed in phosphate buffer [pH = 7.2 to 7.6]
solution for 5 min three times.
• Then the tissue is fixed in 1% osmium tetroxide for 1 h
followed by two to three washing in double-distilled
water.](https://image.slidesharecdn.com/electronmicroscope-241019151852-0f2713d1/85/ELECTRON-MICROSCOPE-pptx-37-320.jpg)









![ULTRATHIN SECTION
• The trimmed blocks are cut further.
• The ultramicrotome is set in an auto mode to have optimum
thin sections.
• Sections less than 100 nm thick are cut. [optimum thickness
is 80 nm]
• The reflected light from the section gives information about
the thickness of the slide.](https://image.slidesharecdn.com/electronmicroscope-241019151852-0f2713d1/85/ELECTRON-MICROSCOPE-pptx-47-320.jpg)