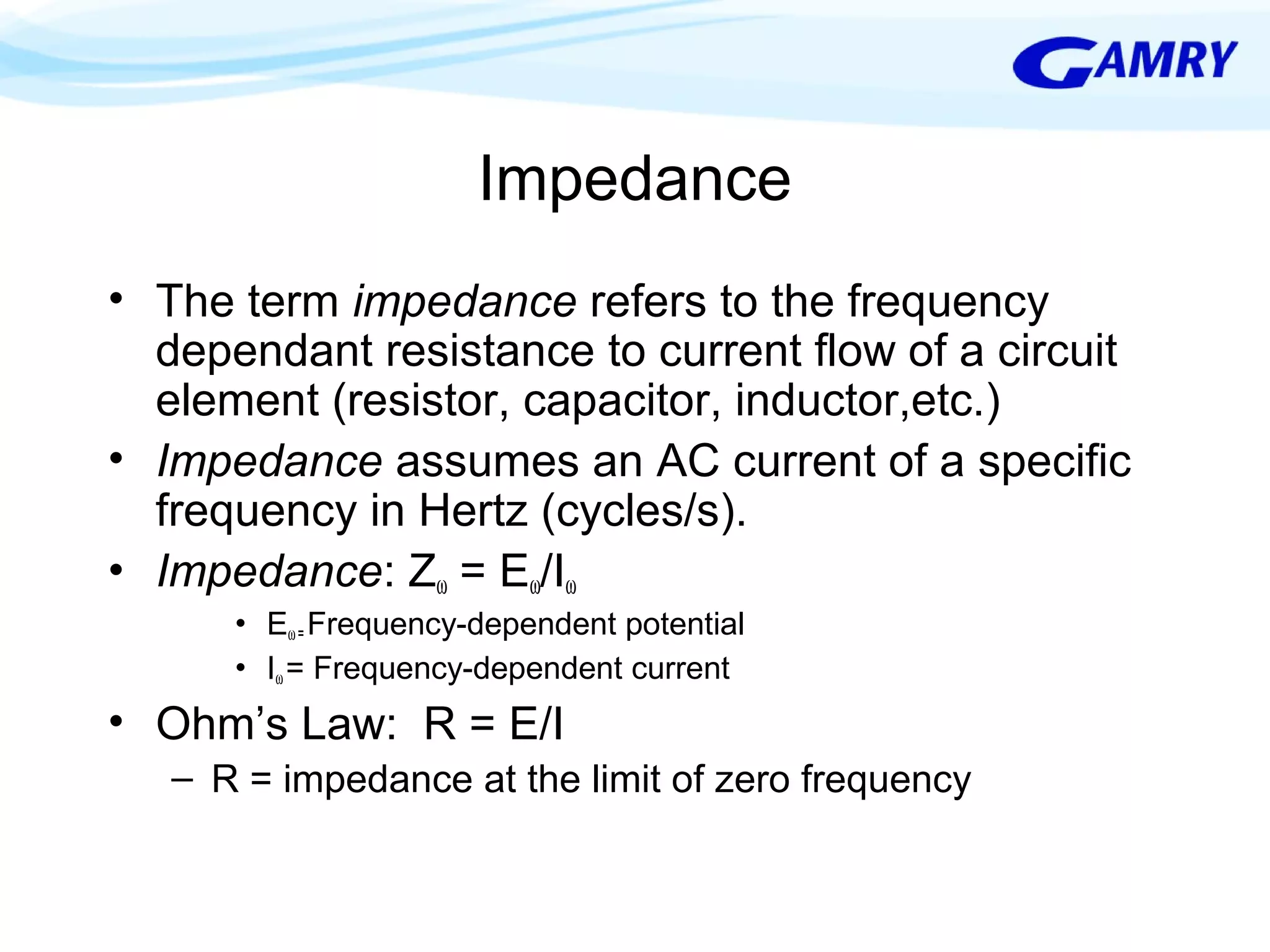
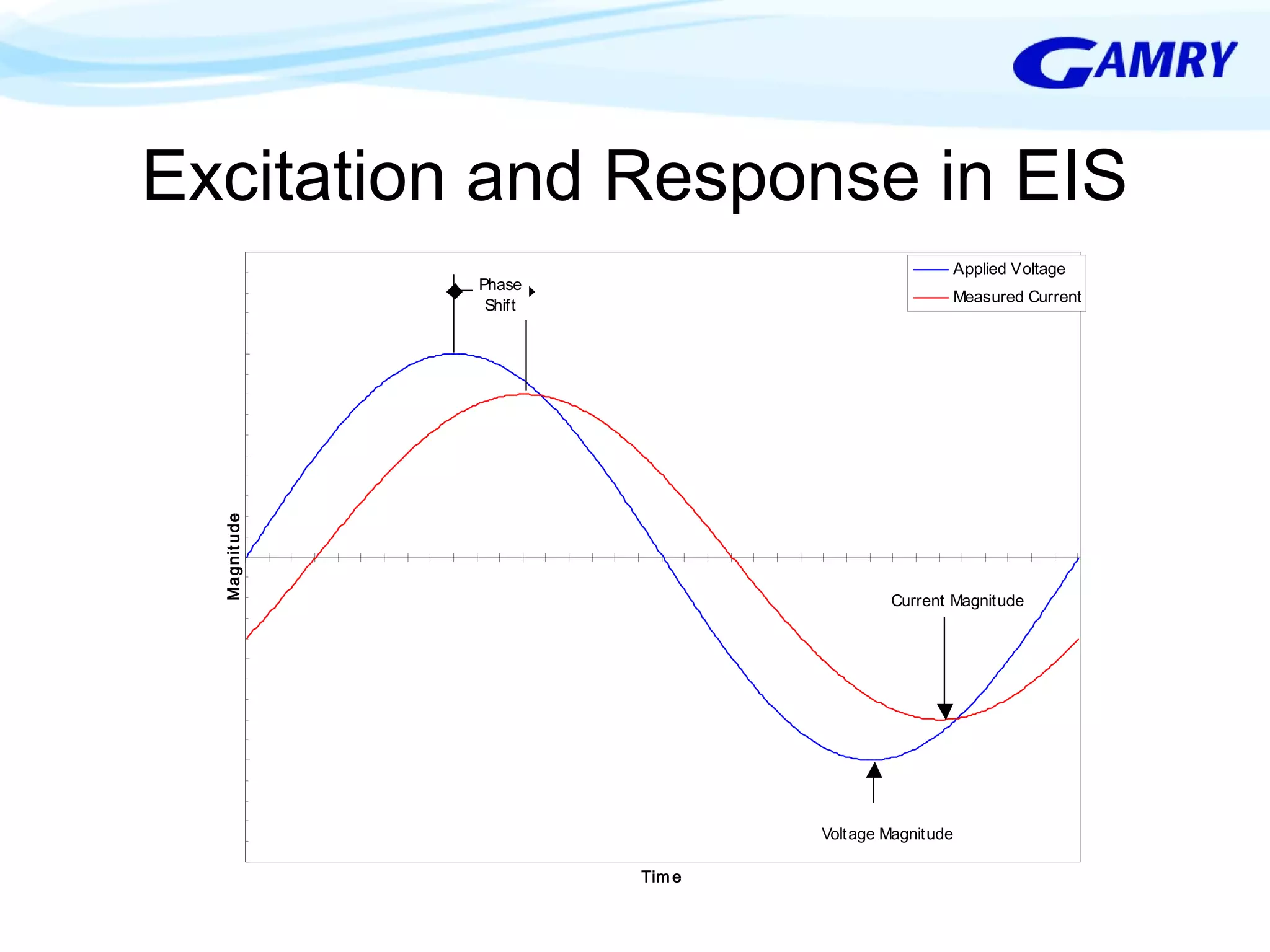
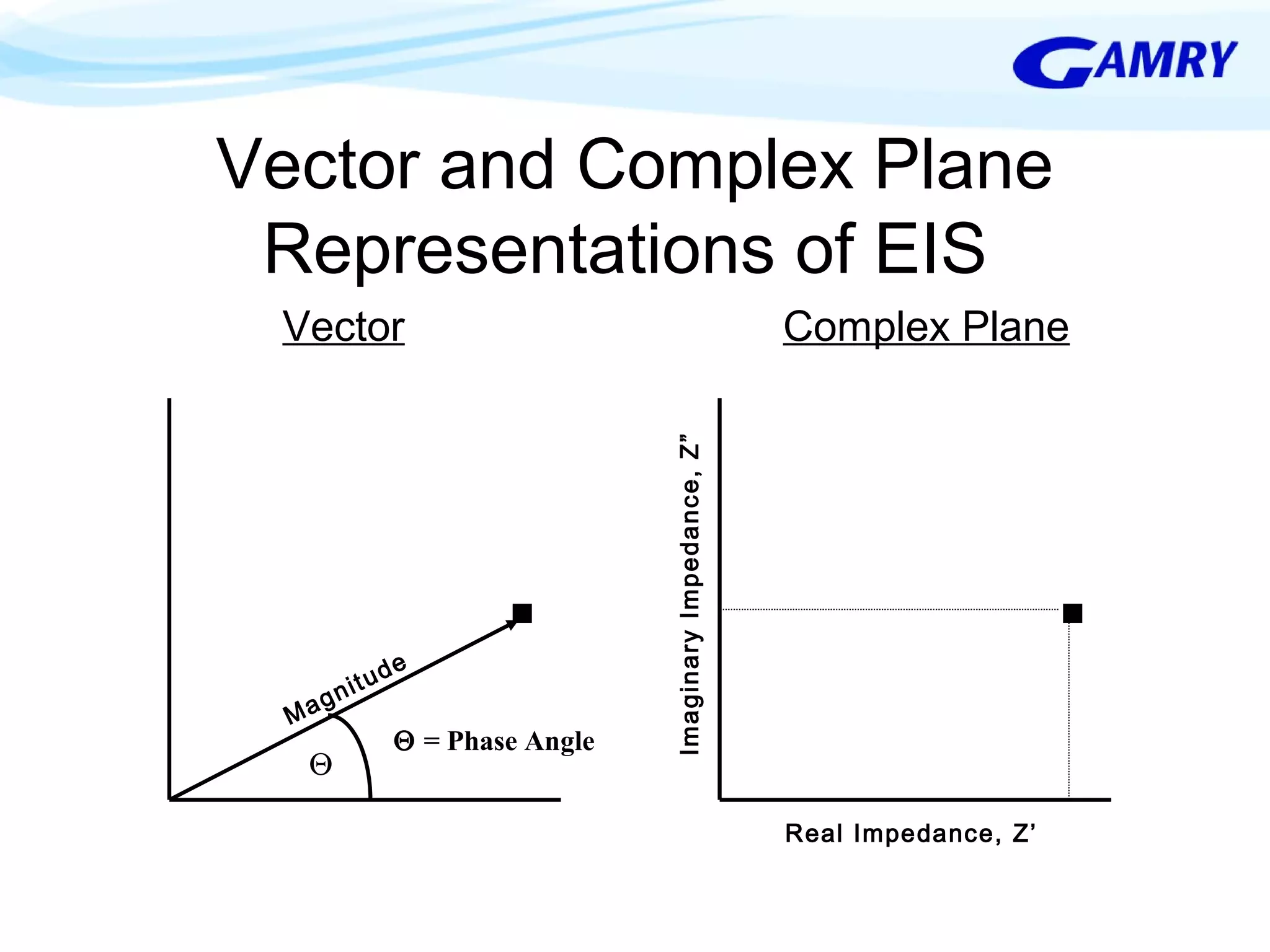
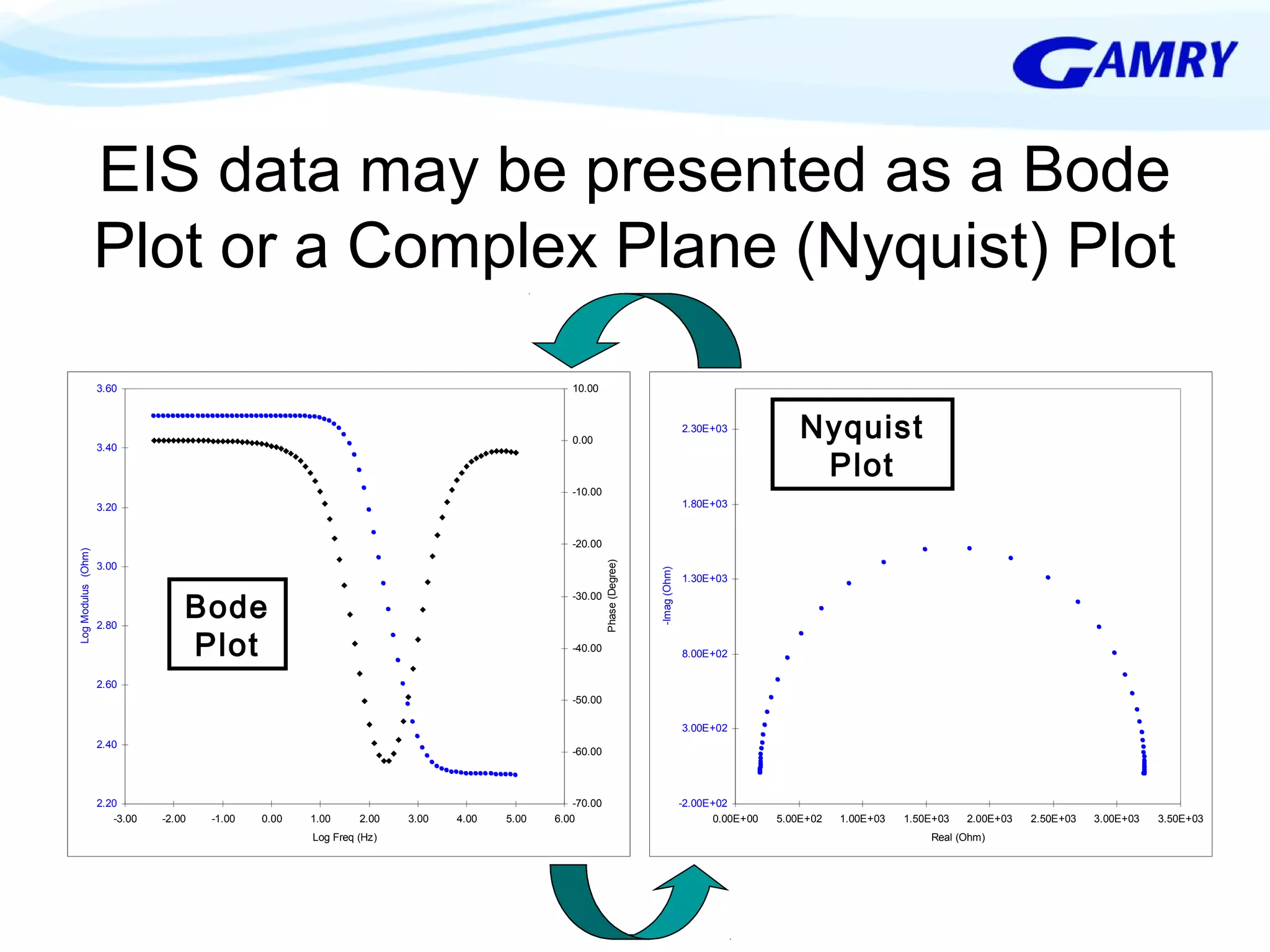
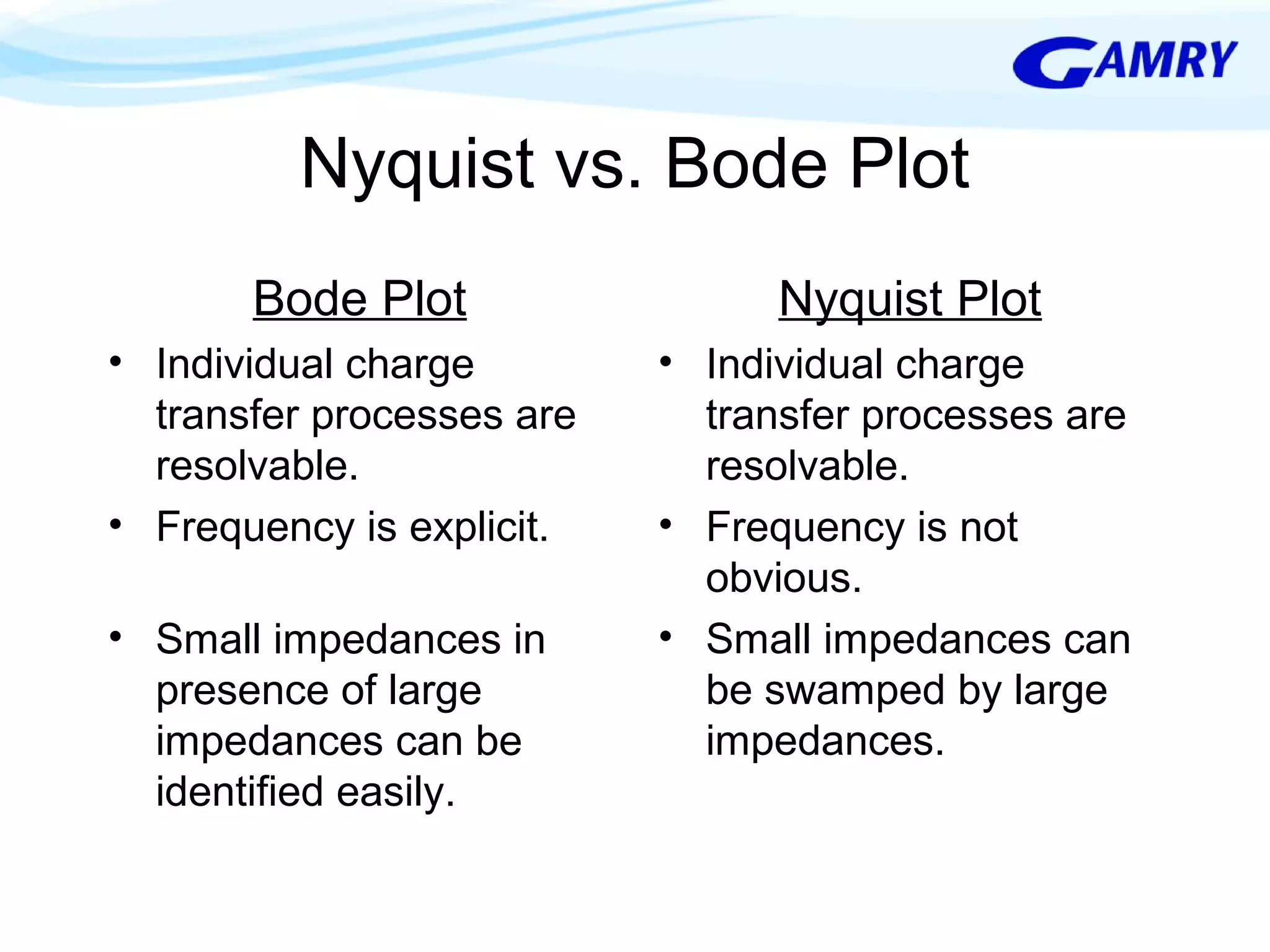
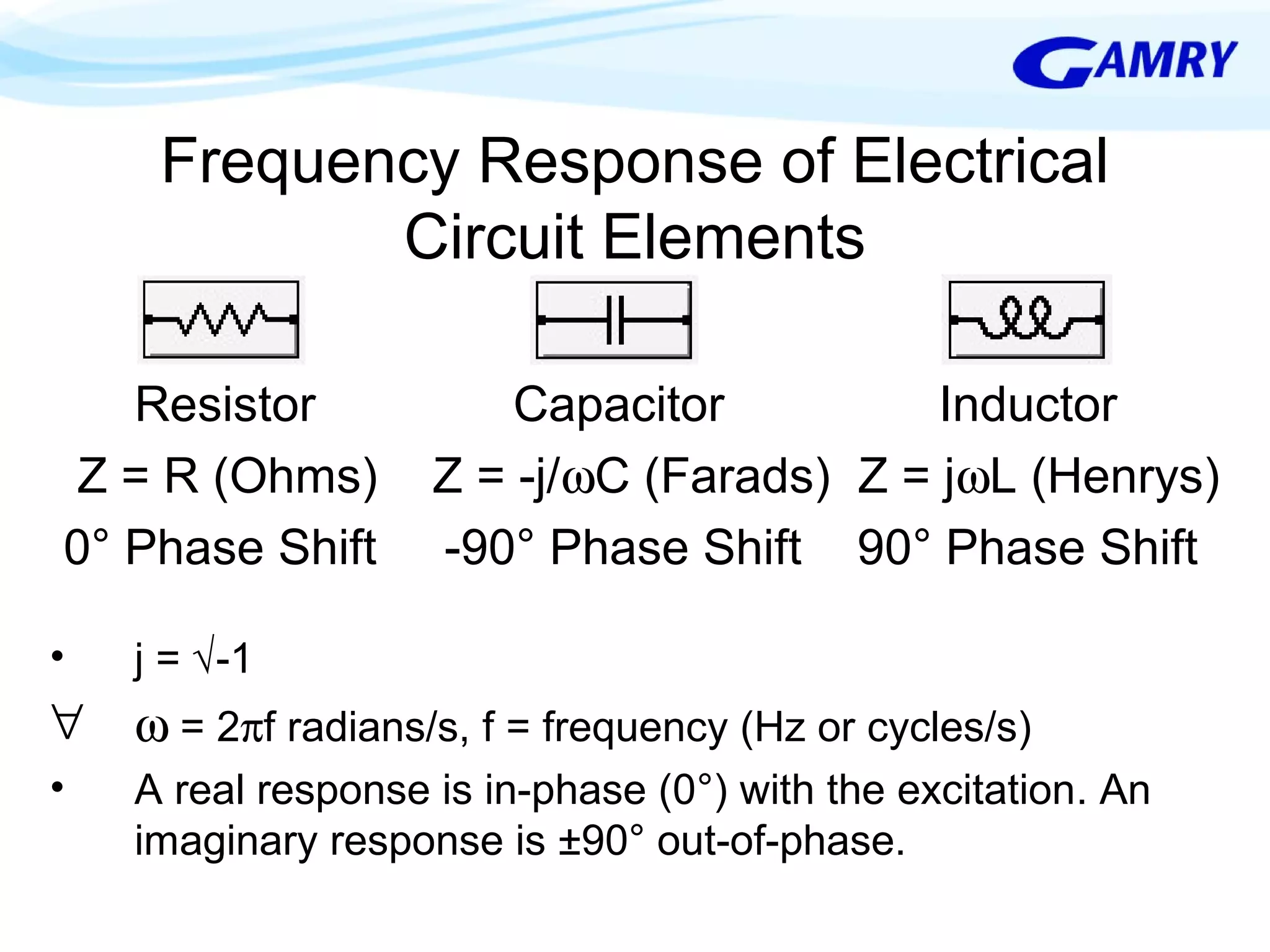
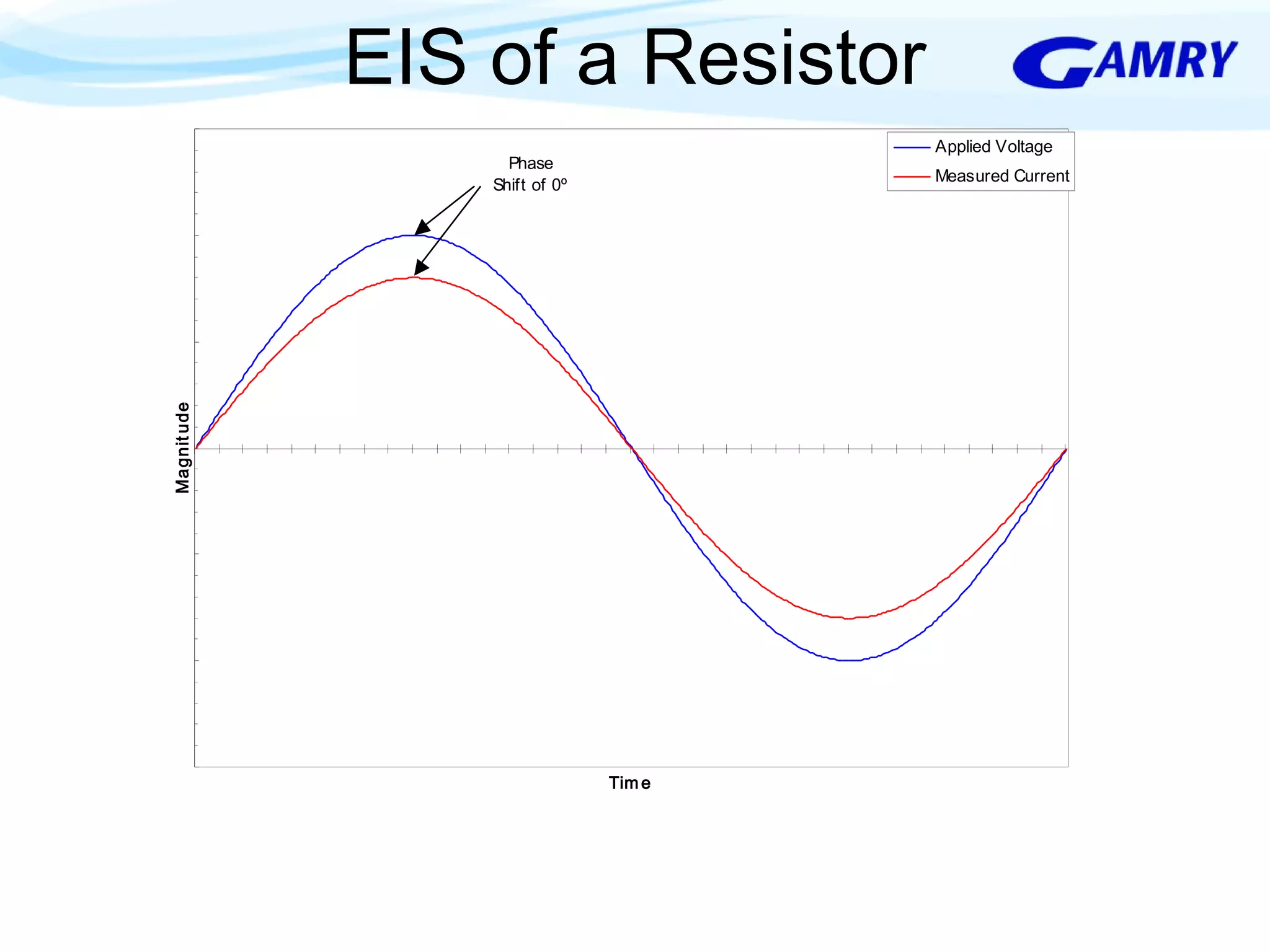
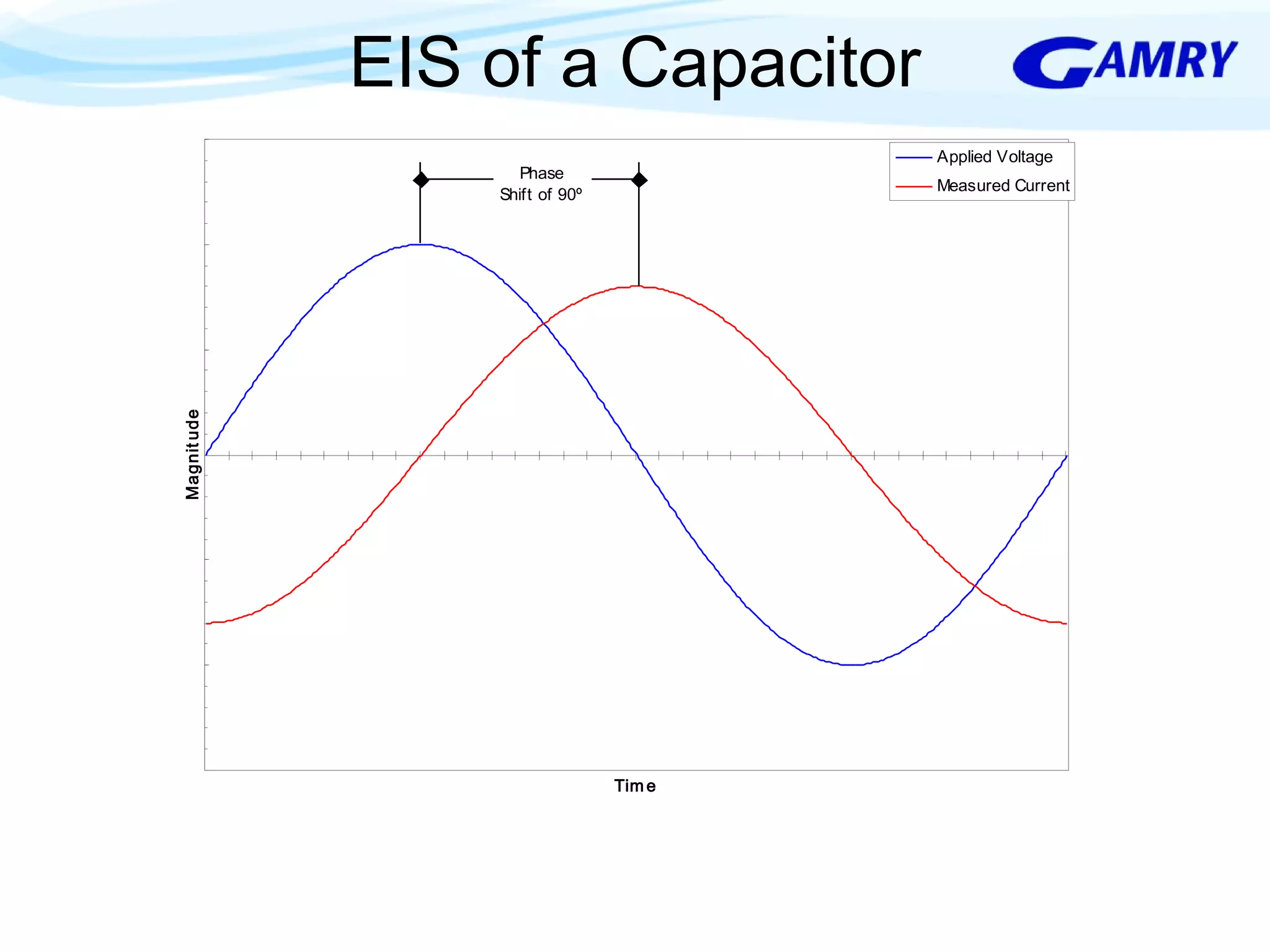
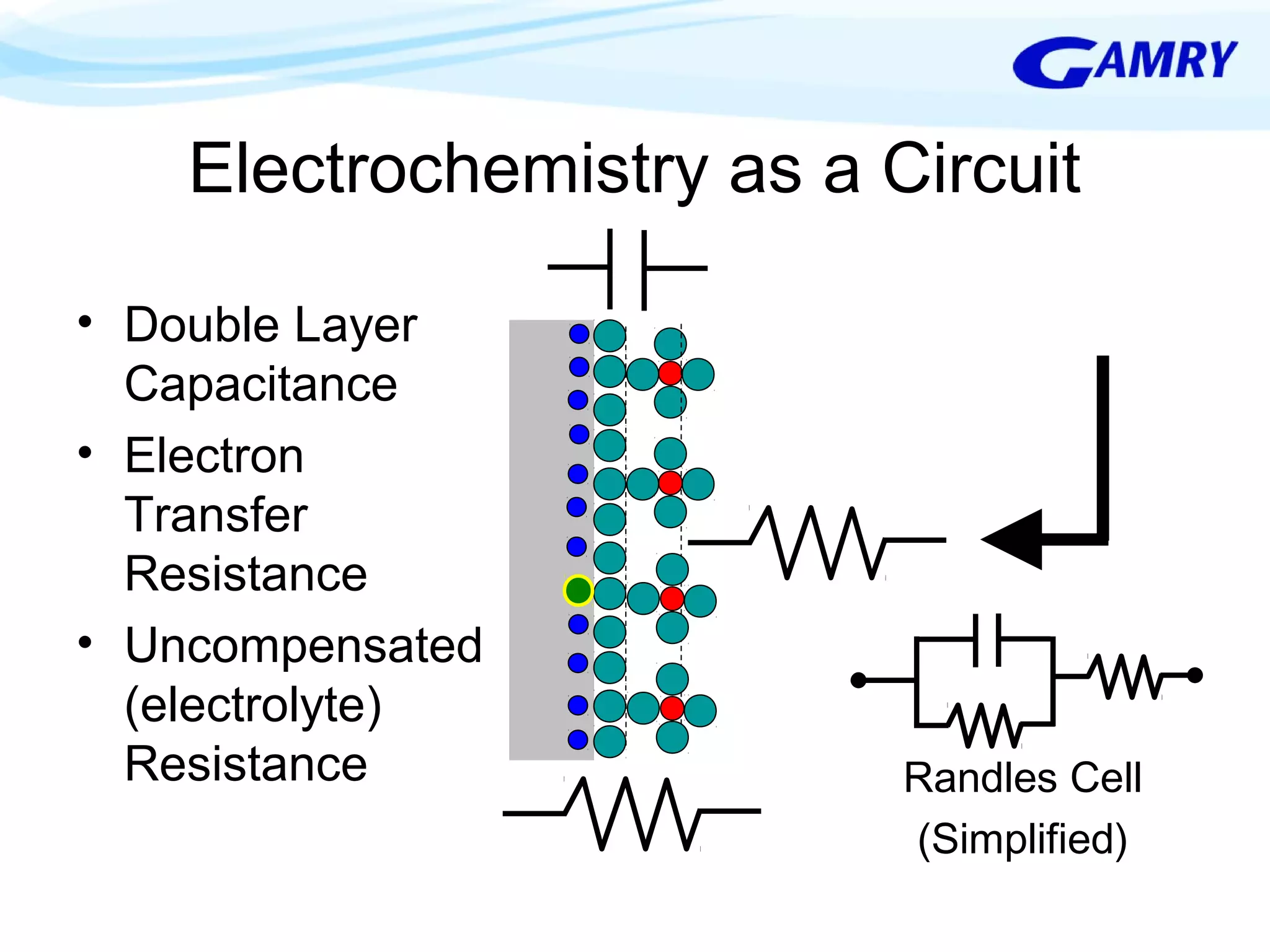
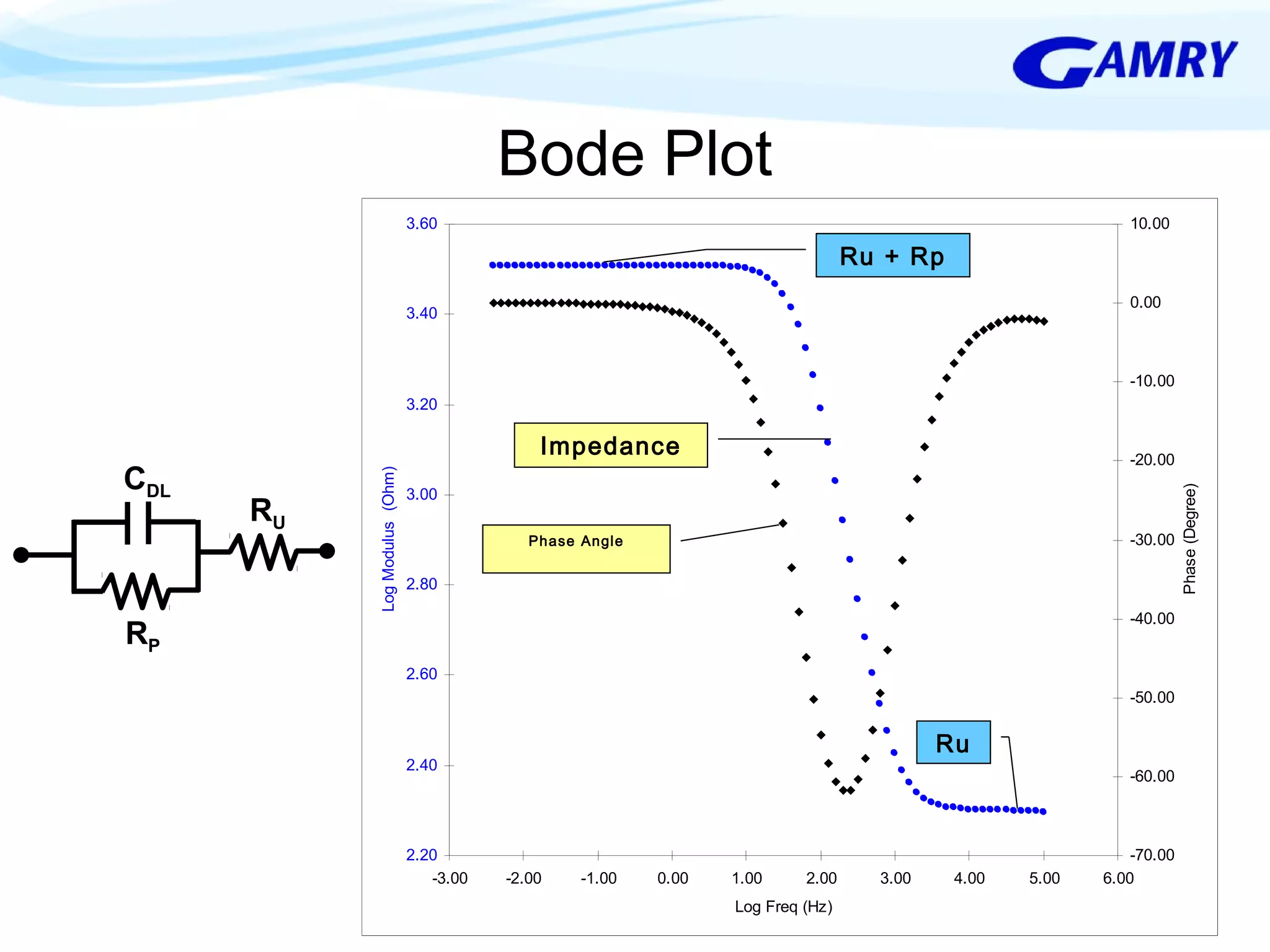
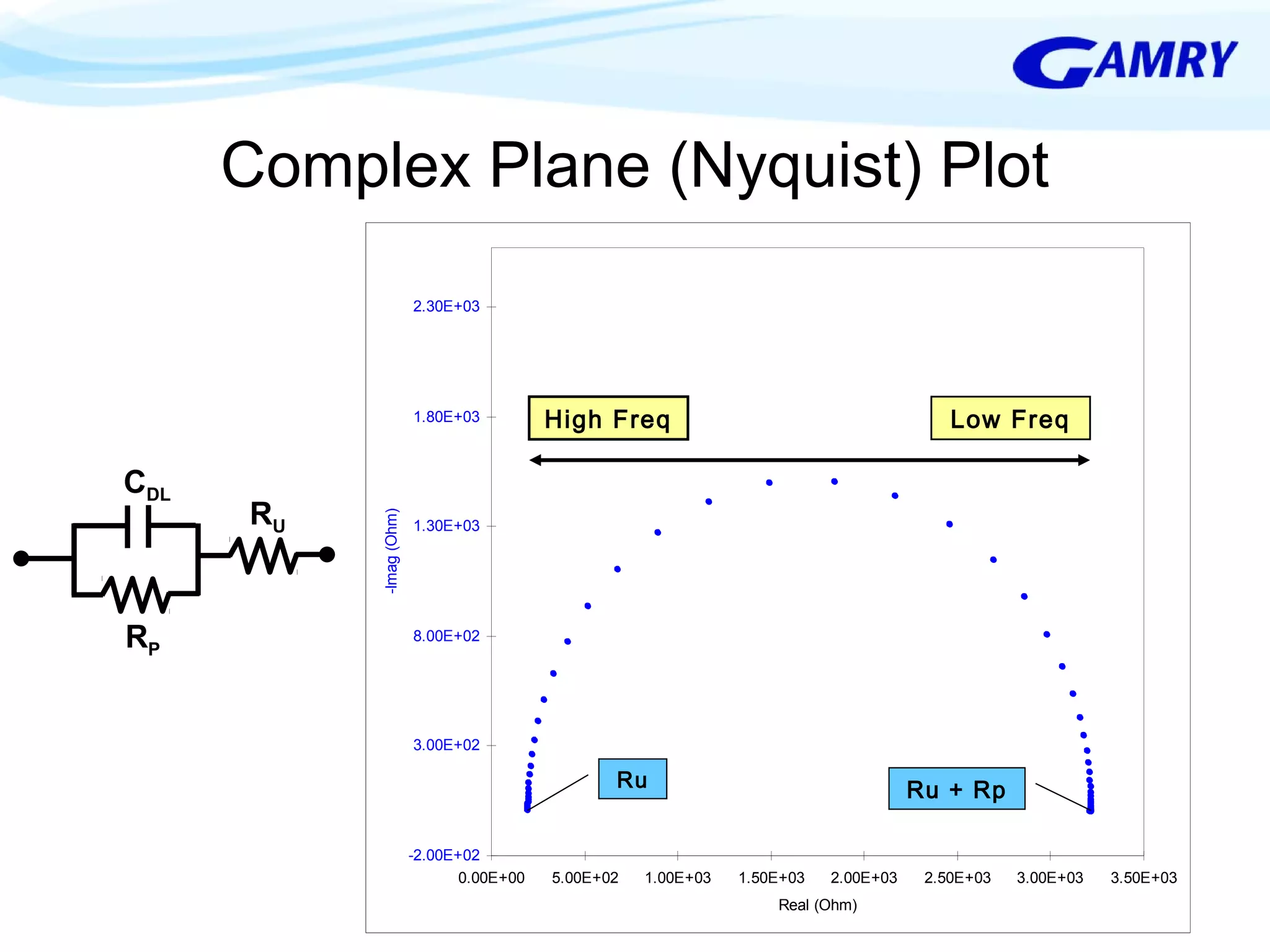
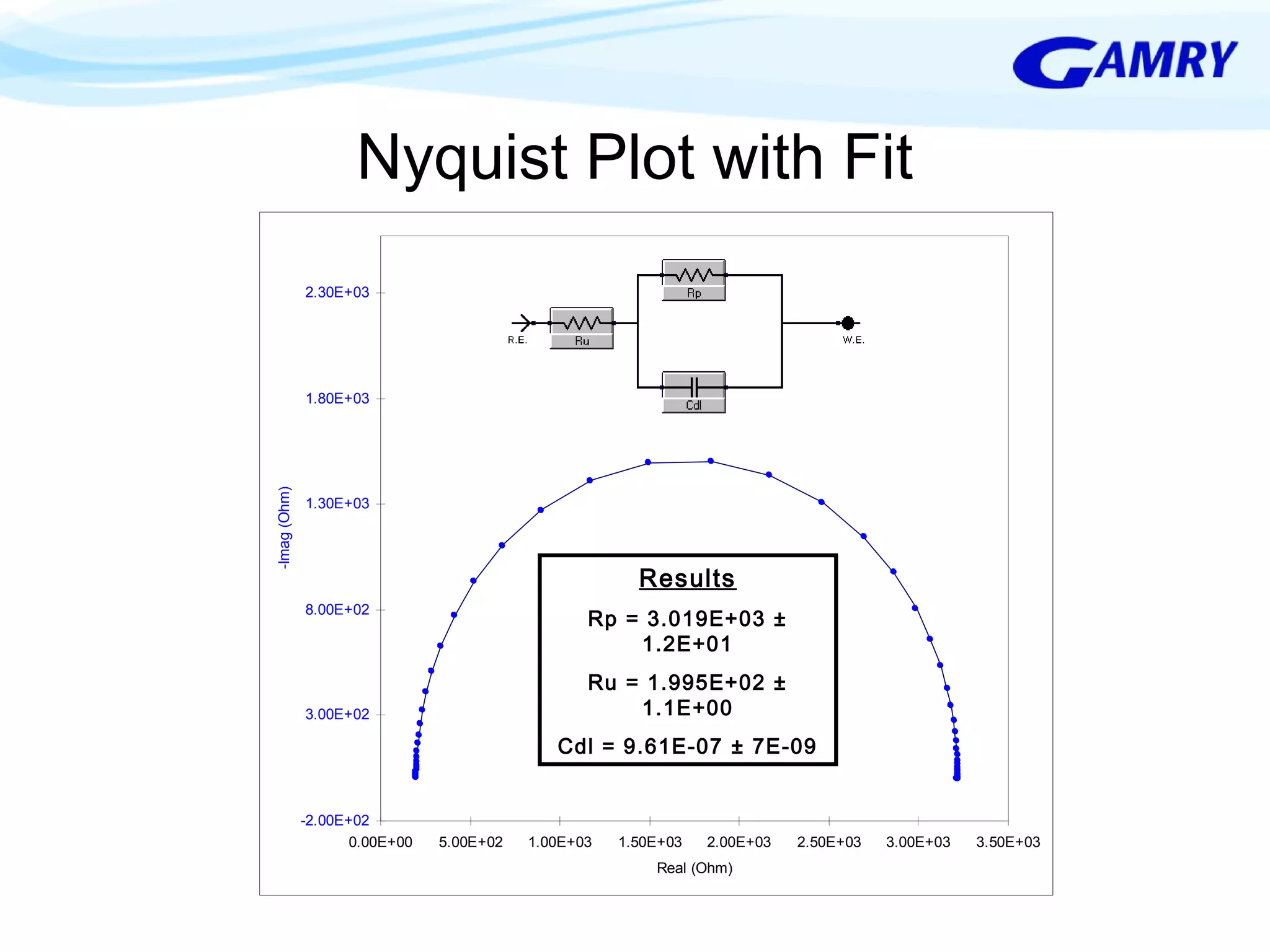
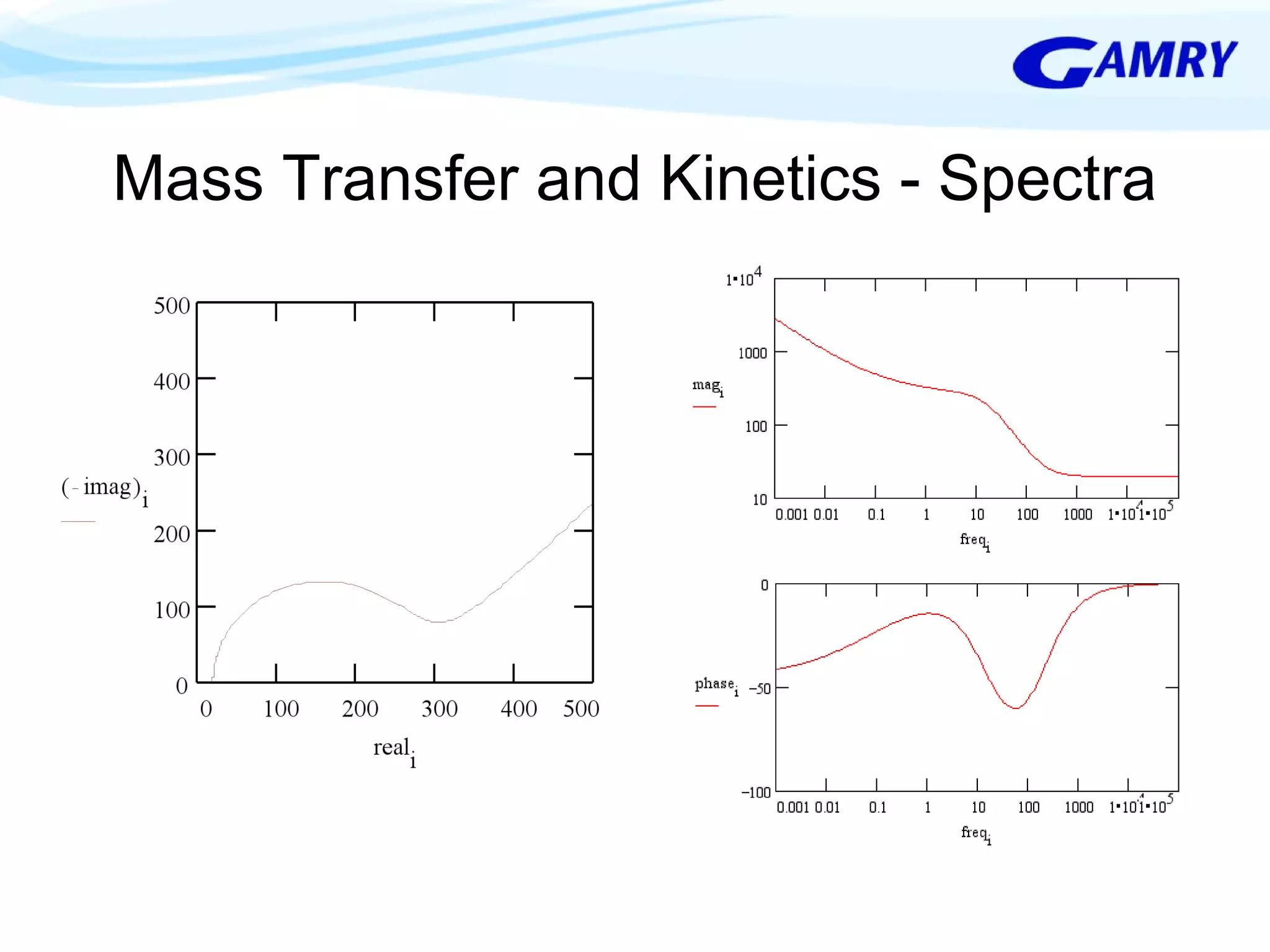
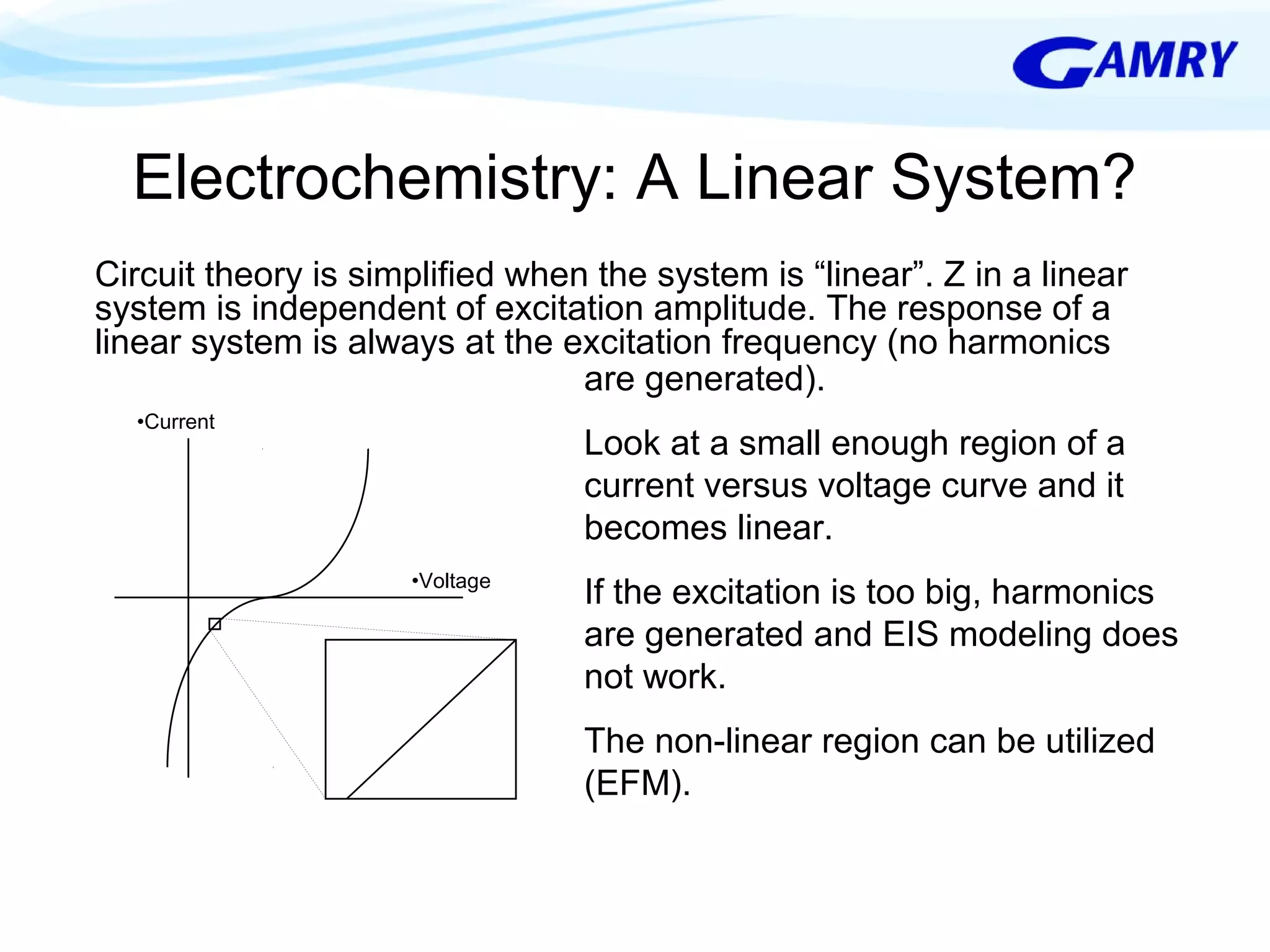
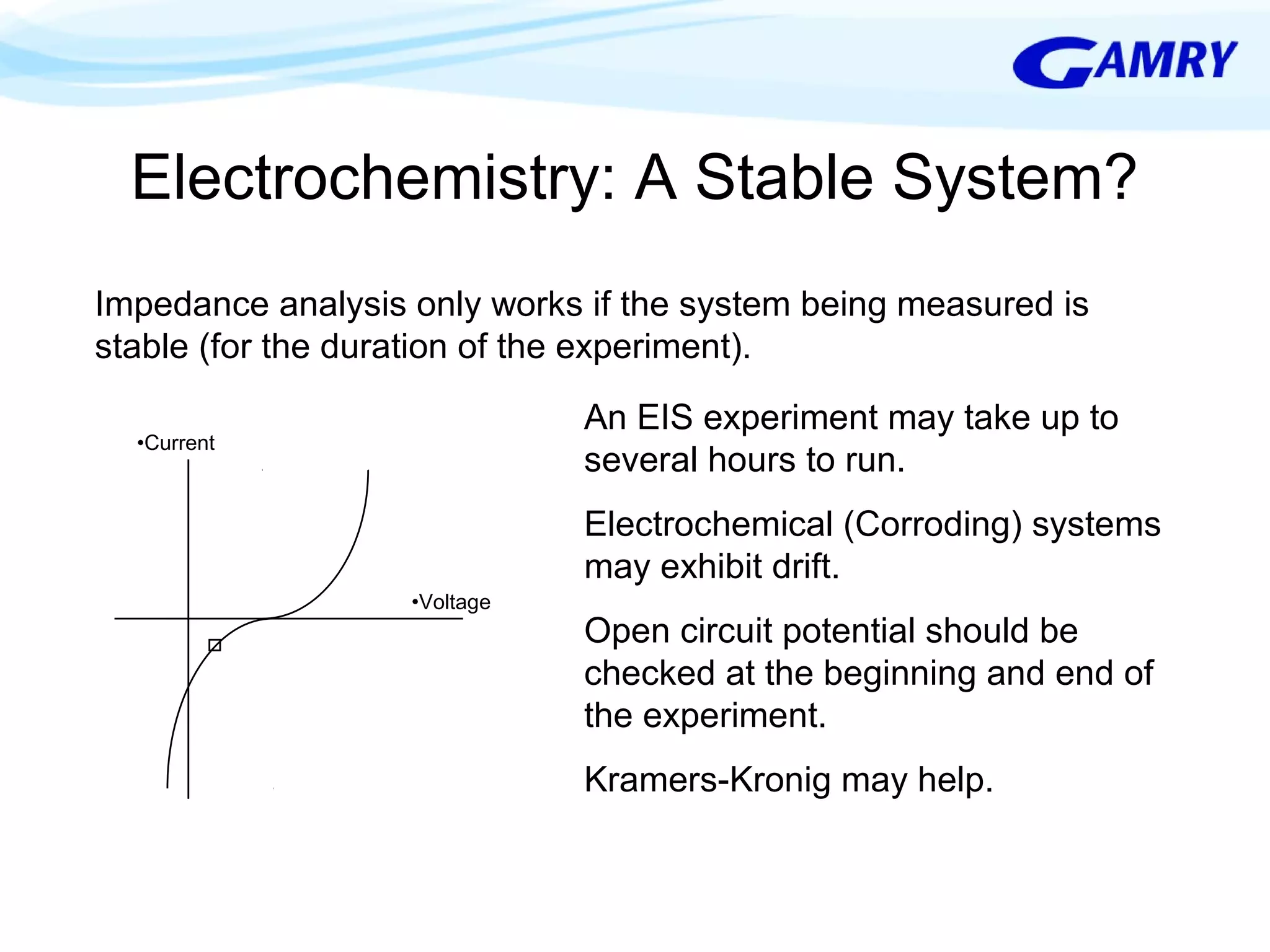
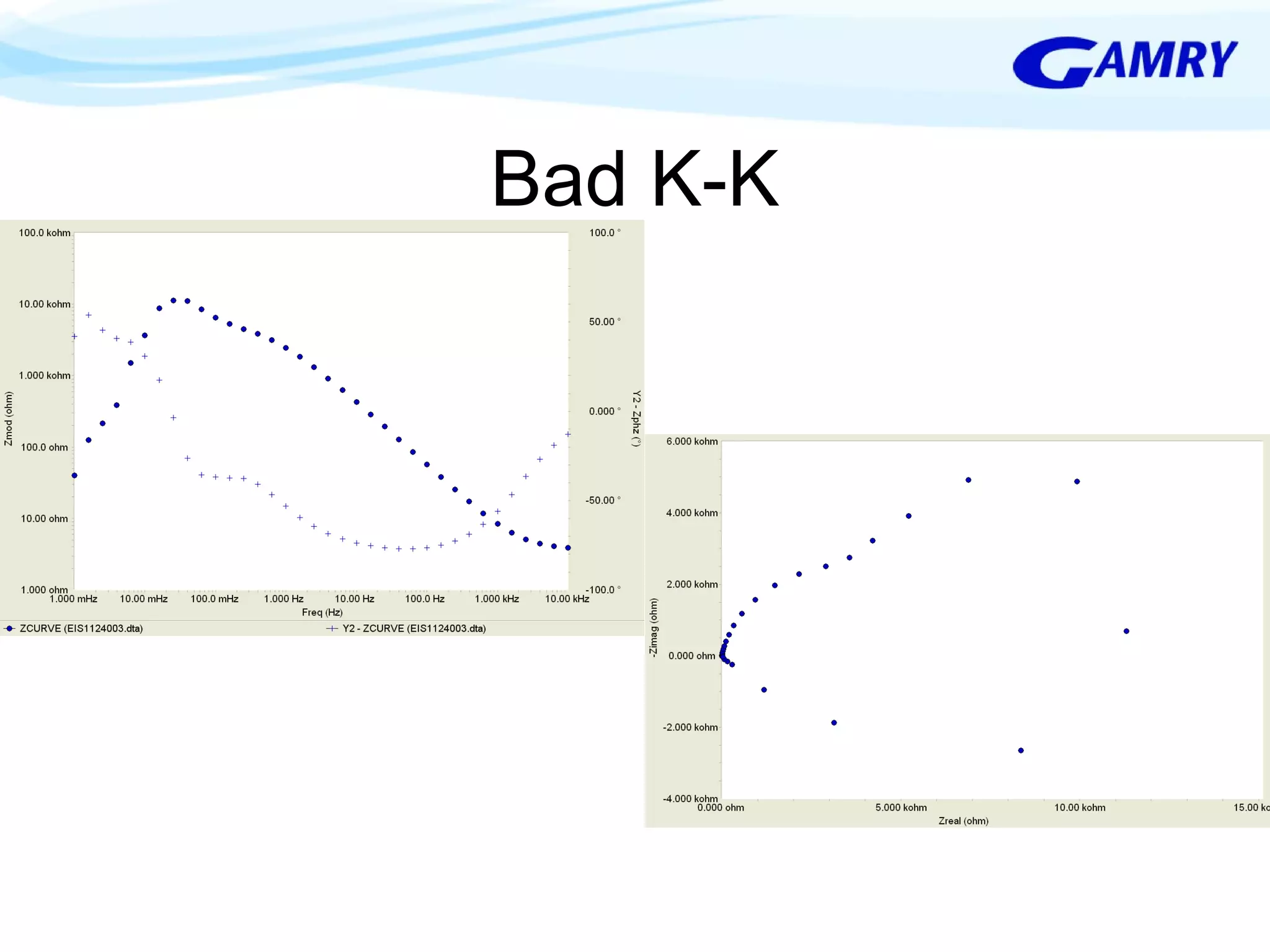
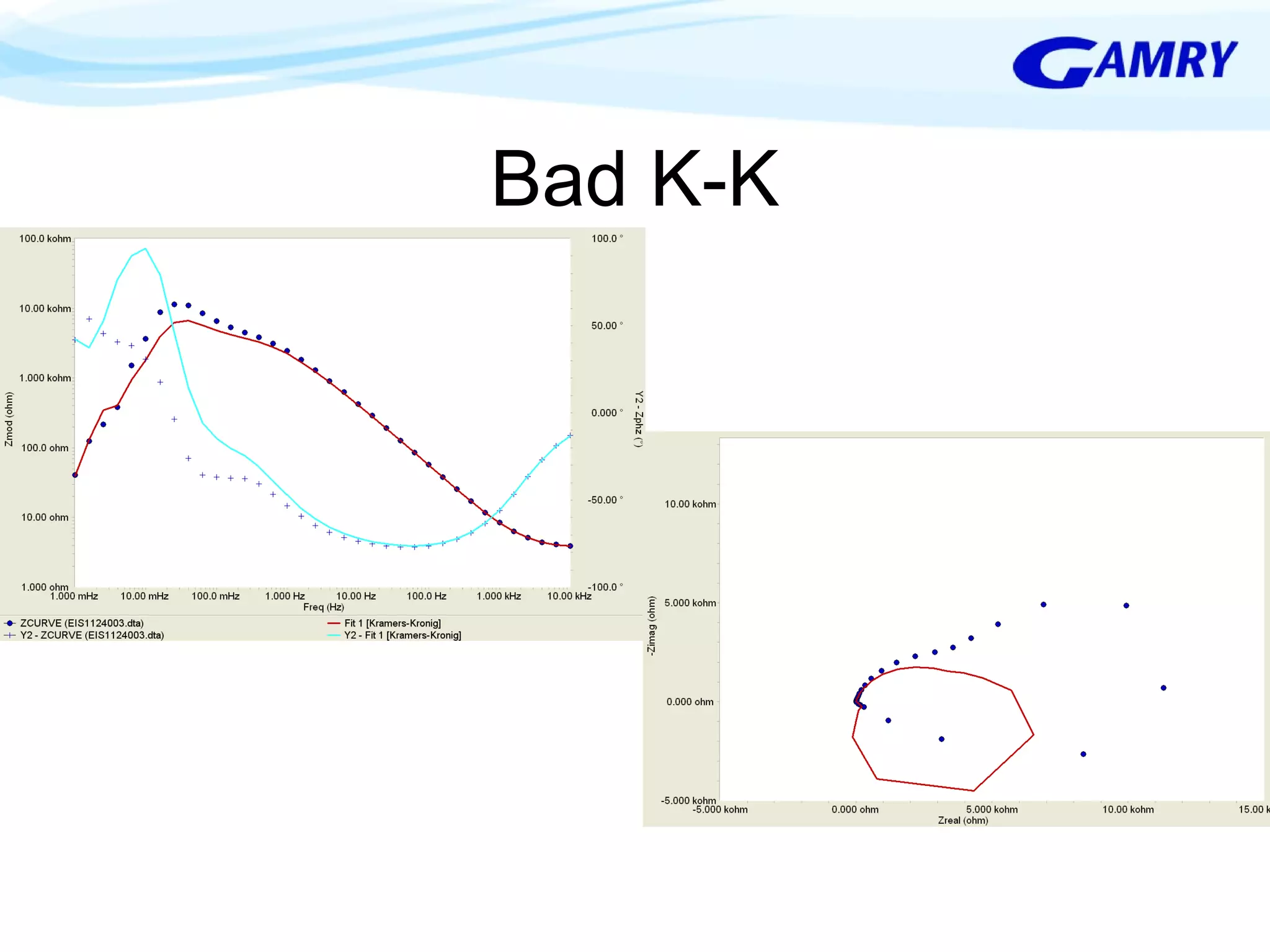
The document provides a comprehensive introduction to electrochemical impedance spectroscopy (EIS), detailing its fundamental concepts, measurement techniques, data presentation, and analysis methods. EIS is highlighted as a powerful, non-destructive evaluation technique that can offer deeper insights into electrochemical reactions compared to traditional methods. It emphasizes the importance of linear, stable, and causal systems for accurate EIS data and outlines necessary instrumentation and analysis steps.