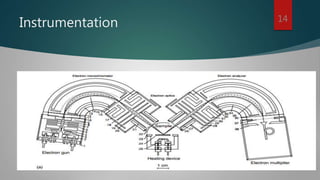
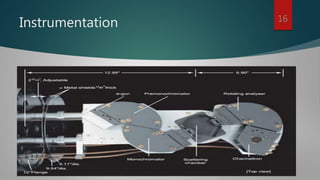
High-resolution electron energy loss spectroscopy (HREELS) is a surface science technique that analyzes small energy losses of low-energy electrons to study vibrational modes of species at surfaces. It provides detailed information about surface interactions, bonding, and adsorbate coverage, focusing on the interactions of electrons and the outermost atomic layers. Despite its advantages in sensitivity and broad spectral range, HREELS requires an ultrahigh vacuum environment and conductive sample surfaces.