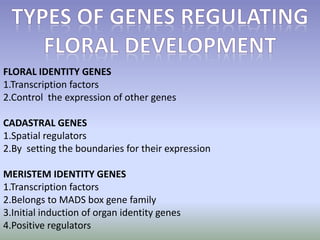
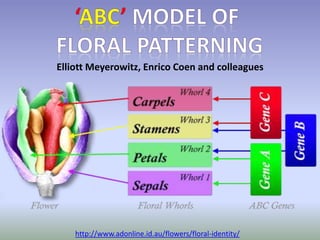
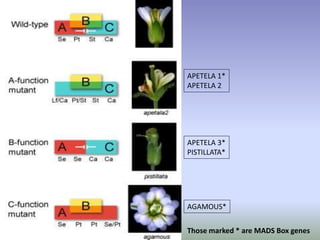
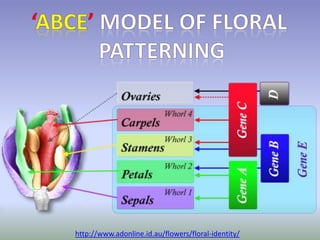
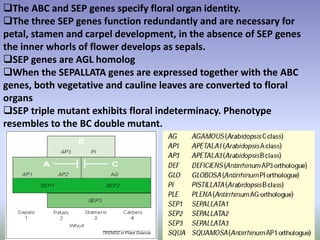
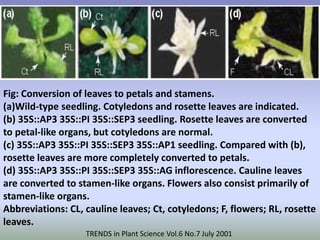
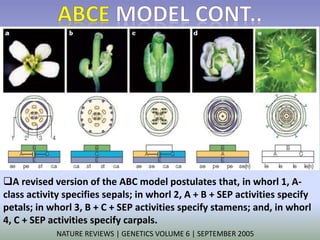
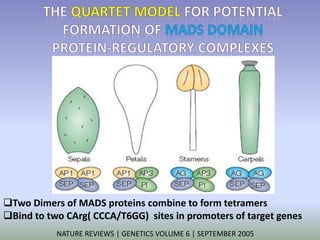
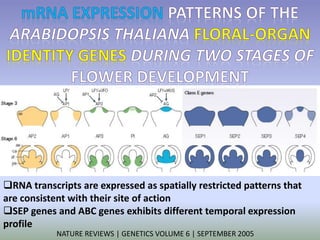
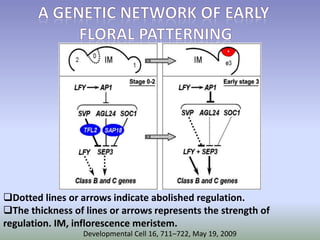
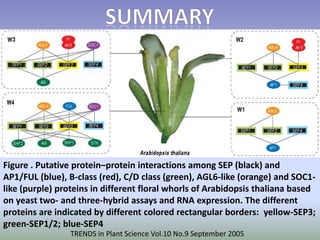
The document discusses floral identity genes in plants. It notes that floral identity genes include transcription factors that control the expression of other genes. Meristem identity genes belong to the MADS box gene family and are involved in organ identity. Key floral identity genes in Arabidopsis thaliana include APETALA1, APETALA2, APETALA3, PISTILLATA, and AGAMOUS. The ABC model of floral organ identity specifies sepals, petals, stamens and carpels based on the combinatorial activities of A, B, and C genes along with SEPALLATA genes.